1. Context
Venous stasis ulcers (VSU) are an advanced and painful manifestation of chronic venous insufficiency (CVI). It is estimated that 1 - 2.7% of the 6 - 7 million CVI patients in the United States develop VSU throughout their illness. Current management of VSU is costly. It is also associated with a poor prognosis, high recurrence rate, poor pain management, and significantly reduced quality of life (QoL) (1-3). Topical volatile anesthetic agents may offer improved pain relief and symptom control in patients suffering from chronic VSU. This review summarizes a topical formulation of volatile anesthetic and its implications for the management of VSU.
1.1. Pathophysiology of Venous Stasis Ulcers
Several theories exist for the molecular pathophysiology of VSU. One theory suggests that interrupted blood flow via venous hypertension mediated capillary damage to the submucosa and more superficial layers of skin results in hypoxia and decreased nutrients to the skin that subsequently causes ulcer formation from a damaged dermis. This theory, however, has been challenged by recent microcirculation studies (4, 5). An alternative theory highlights the inflammatory changes that result from red blood cell extravasation secondary to venous hypertension. It is believed that red blood cell extravasation into the dermis triggers an inflammatory cascade, eventually leading to transforming growth factor beta (TGF- B) production, which triggers dermal fibrosis lipodermatosclerosis, and eventually ulceration (4).
Regardless of the exact pathophysiology, it is agreed that the inciting event for stasis ulcers is venous hypertension secondary to valvular dysfunction, flow obstruction, and insufficiency of the calf muscle pump (reduced activity/mobility), or some combination of these factors - most presenting as chronic edema. As such, it is unsurprising that the leading cause of VSU is CVI. Other conditions that also present as chronic edema, venous hypertension, and eventually lead to ulcer formation include congestive heart failure (leading to increased right heart pressures and distal fluid buildup), increased central obesity (and the presence of a large abdominal pannus), and post-thrombotic syndrome following deep vein thrombosis (6).
1.2. Epidemiology and Psychosocial Impact
In the US, an estimated 1 - 2% of CVI patients (of 6 - 7 million nationwide) develop at least one ulcer throughout their illness. Of these, approximately 40% of patients then develop subsequent ulcers, making VSU prognostically poor (1). In addition to their high prevalence, venous stasis ulcers also account for a sizeable share of healthcare expenditure and loss of productivity in the US. Venous stasis ulcer treatment currently costs around $2.5 billion/year and results in nearly 2 million workdays lost annually (4).
VSUs are also associated with significant pain and a marked decrease in quality of life in both physical and psychological contexts. Several studies have noted that patients with recurrent stasis ulcers report moderate to severe symptoms, including intense, frequent, and uncontrolled pain, lost productivity, social isolation, anger, depression, negative self-image, and impaired mobility (2, 3, 7). VSUs are not only epidemiologically significant while also severely harming patients’ physical and mental well-being.
2. Clinical Presentation
VSUs are currently diagnosed by careful examination of patients who present with signs of CVI. In history, common findings include feelings of heaviness, itching, tingling, and restless legs, that typically worsen at the end of the day (4). There also exist skin changes that usually precede the development of an ulcer that can be used as red flag symptoms for closer monitoring of said patients, including edema, varicosities, severely blanched skin lesions, dermal atrophy, and possible hyperpigmentation or lipodermatosclerosis, most commonly overlying the medial malleolus (1, 4).
Thorough evaluation typically goes beyond the history and physical examination, incorporating imaging modalities such as venous reflux studies and duplex ultrasonography (with insufficiency as reflex > 0.5 with distal compression). Ulcer location, size, and degree of necrosis should be carefully noted, and any signs of dramatic change should be suspected for infection or flagged for biopsy, as squamous cell carcinoma (Marjolin ulcer) is a common complication of untreated or treatment unresponsive stasis ulcers (1, 4, 6).
At the time of diagnosis, venous stasis ulcers can also be classified using the American Venous Forum Clinical-Etiological-Anatomical-Pathophysiological (CEAP) classification (Table 1).
| Class | Clinical Features |
|---|---|
| C0 | No visible or palpable signs of venous disease |
| C1 | Telangiectasias or reticular veins |
| C2 | Varicose veins |
| C3 | Edema |
| C4 | Skin changes, i.e., lipodermatosclerosis |
| C5 | Healed ulceration |
| C6 | Active ulceration |
American Venous Forum CEAP Classification of Venous Stasis Ulcers
Importantly, symptomatic improvement in ulcer management tends to center on pain management, as pain is often cited as the most debilitating symptom in patients with stasis ulcers (2, 3). Several studies and reviews have shown that patients with venous stasis ulceration report high levels of frequent, undertreated, and debilitating pain (2, 8, 9). A survey study of patients with venous stasis ulcers found that on a 6 point pain scale, 64% of respondents reported a pain score of either 4 (horrible) or 5 (excruciating) and felt that their pain was undertreated (10).
Another multi-center, cross-sectional study of 381 patients with venous ulcers of various etiologies reported pain outcomes using the short form survey (SF-12) questionnaire and a visual analog scale (VAS). This study found higher mean VAS scores for ulcers that were more chronic and recurrent (mean VAS of 53.8, 53.9, 62.9 for ulcers of over 12 mo. duration, vs. 38.7, 36.9, 46.8 for ulcers of 3 mo. duration) and was larger in surface area. More chronic and longer duration ulcers were also associated with a lower SF-12 score, indicating lower quality of life in these individuals (11).
Unresolved chronic pain has been shown to decrease treatment adherence in patients with stasis ulcers and decrease quality of life, as previously discussed (11). Pain is especially exacerbated in patients with VSU when they undergo dressing changes or wound cleaning. Current treatment options include topical lidocaine, prilocaine, or oral analgesics (e.g., NSAIDs, acetaminophen, metamizole, tramadol, and opioids) - none of which have been proven to satisfactorily abate pain in venous stasis ulcer patients. Patients that rely on analgesics with high addictive potentials, such as opioids or tramadol, are also at risk for developing dependence and the numerous side effects associated with each of these substances. These factors point towards an unmet need for safe, efficacious pain medications in this patient population.
2.1. Current Treatment Options
Current guidelines for the treatment of stasis ulcers advocate for conservative treatment with compression, elevation, proper wound management (including bandaging), and exercise to improve the efficiency of the calf muscle pump. Treatment-resistant patients are often referred for surgical procedures, which include sclerotherapy, endovenous thermal ablation/stripping for superficial veins, and valve reconstruction or a transplant for deeper veins (1, 4, 8). Treatment regimens are modified for patients with overlying arterial disease.
There is also a growing body of evidence for the pharmacological management of VSU. Topical hydrogen peroxide application, for instance, was found to reduce ulceration and improve perfusion when compared with controls (12). Additionally, tumor necrosis factor alpha (TNF-alpha) inhibitors, oxerutins, ventropes like micronized purified flavonoid fraction (MPFF), and natural chemicals, including French maritime pine bark extract (Pycnogenol) and horse chestnut seed extract therapy, have all been found to be efficacious (13-18).
In cases where patients are suspected of having superimposed bacterial infections on their ulcers, aggressive wound care and antibiotic use are suggested. Interestingly, studies have not found any efficacy in prophylactic antibiotic use to prevent infections (4, 19). Finally, in cases of progression to stasis dermatitis, the use of steroids to control inflammation is recommended (19).
The goals of all treatment regimens described above are symptomatic improvement, ulcer healing, and avoiding recurrence.
2.2. Topical Sevoflurane for Chronic Venous Ulcers
Sevoflurane has been clinically used to induce and maintain anesthesia for decades, and its safety and clinical efficacy have been investigated amongst different patient profiles in many studies (20, 21). Although the primary route of administration of sevoflurane is the inhalation of the volatile formulation for systemic anesthesia, there are recent studies that explore the local effects of sevoflurane as a topical agent in patients with chronic venous ulcers. Due to its efficacy in inducing local analgesia and promoting wound healing, topical sevoflurane can provide a combined solution to the unmet needs in safe pain management techniques and treatment agents for chronic venous ulcers. With further investigation and approval of the topical formulation, sevoflurane can address the high economic and epidemiologic burden caused by VSU.
2.3. Volatile Anesthetic Mechanism of Action
The mechanism of action of inhaled volatile anesthetics remains unknown (22). Several receptors, including GABA, glycine, and glutamate receptors in the CNS, have been indicated in its effects (23, 24). All have negligible biotransformation and metabolism and therefore are cleared in unchanged form. Sevoflurane specifically undergoes rapid metabolism into fluoride and hexafluoro isopropanol (HFIP) that immediately gets glucuronidated; however, the overall extent of metabolism, in one study, was only 5% and did not near toxic ranges for these metabolites (25).
Sevoflurane, as a topical anesthetic, has been sparsely studied but remains a candidate for local use. The topical formulation of sevoflurane has an identical molecular structure to the volatile form. It is an ether derived molecule that is highly fluorinated (26). Inhaled anesthetics through a subcutaneous injection have been shown to produce a concentration-dependent, localized, and reversible cutaneous analgesia, which attained complete analgesia at high concentrations (27). Several mechanisms have been proposed for these analgesic effects. One proposed mechanism indicates a possible direct inhibitory effect on vascular smooth muscle, thereby decreasing vascular flow (28). There has also been evidence of volatile anesthetics suppressing sodium channels and activating subunits of voltage-dependent potassium channels in central and peripheral mammalian cells (29). Increased potassium conductance decreases neuron excitability and could create changes in voltage-gated sodium channels (30). Sodium channel inhibition leading to reduced transmission of neural signals relates the mechanism of volatile anesthetics to that of local anesthetics, which have known analgesic mechanisms of effect.
In human trials, sevoflurane used topically was shown to increase responses to electrical stimuli; however, it has also been shown to attenuate mechanical stimuli (31, 32). This may be explained similarly to fiber sensitivity to local anesthetics. Cutaneous C fiber nociceptors are not sensitized by mechanical stimulation, A-delta (Ad) fibers respond to mechanical stimuli only, and both electrical and mechanical stimulation sensitizes A beta (Ab) afferents at non-noxious levels (31). Ad fibers have much faster conduction velocities at 5 – 40 m/s compared to 0.5 – 2 m/s of C fibers with non-significant differences in fiber diameter (33). Ad fibers, therefore, may be preferentially inhibited by topical sevoflurane. However, at high concentrations, a full analgesic effect can be seen. Motor neuron response in isolated rat pup spinal cords to single, repetitive C fiber stimulation was completely abolished action potentials with 250 µg of sevoflurane, causing both analgesia and paralysis (34).
Separately, pain can be caused by bacterial-derived growth factors directly influencing membrane receptors leading to pore assembly and calcium ion influx, causing nociceptive fiber activation and sensitization as a contributory mechanism to their survival (35). Isoflurane and halothane have been shown to suppress T calcium current in dorsal root ganglion cells and adrenal glomerulosa cells (36). Through calcium channel inhibition, volatile anesthetics may play a role in analgesia by dampening neuronal excitability and inhibiting direct bacterial nociceptor activation and having antimicrobial effects.
3. Clinical Use
Although sevoflurane is still a novel therapeutic agent with disputed mechanisms of action, a body of literature has started to build around its utility in several use cases. Table 2 highlights the findings from several publications in the last decade that make a case for the inclusion of topical sevoflurane as a critical analgesic and wound management agent in patients with CVI induced ulcers. Below, these findings are further elucidated, as are recommendations for the use of sevoflurane and other topical volatiles for off label uses. Details on safety, efficacy, and suggested administration of sevoflurane are also included.
| Author (s) | Title | Methods/Type | Conclusions |
|---|---|---|---|
| Skouteri et al. (31) | Local application of halothane, isoflurane or sevoflurane increases the response to an electrical stimulus in humans. | Randomized cross over study; Double blinded; N (total) = 70; Experiment 1: 30 subjects received 1 mL halothane, 1.5mL isoflurane, 2.7mL sevoflurane on one forearm in randomized order and equal volume of water on the other forearm. Experiment 2: 20 subjects received 2 mL, 4 mL, and 6 mL halothane one forearm and equal parts water on the other in randomized order. Experiment 3: 20 subjects received 5 mL sevoflurane on one arm and 5 mL water on the other.all experiments studied pain perception response via VAS score from an 60 ma peripheral nerve electrical stimulus | Low doses of all three volatile anesthetics increased pain response to electrical stimulus. High doses of halothane and sevoflurane had no effect on pain response to electrical stimulus. |
| Srivastava et al. (37) | Comparative evaluation of volatile anaesthetic agents for attenuation of venous cannulation pain: a prospective, randomized controlled study | Prospective randomized control study; Placebo controlled; double-blind N (total) = 120; The analgesic effects of sevoflurane, halothane, and isoflurane were compared at equipotent doses based on the mac of volatile formulation (2.7 mL, 1 mL, 1.5 mL respectively). The placebo group received 2.5 mL of water. The two outcomes that the study measured was the incidence of pain and VAS scores during venous cannulation. | Significant decreases in both incidence of pain and VAS scores were observed in the halothane treated group compared to control, sevoflurane and isoflurane groups. |
| Fernández-Ginés et al. (26) | Efficacy and safety of topical sevoflurane in the treatment of chronic skin ulcers. | Prospective observational study; Single-blind; N (total) = 15 patients with chronic venous ulcers were randomly assigned to receive sevoflurane treatment (1 mL per cm2 of ulcer area 1 - 4 times daily) and standard wound care (ulcer cleaning, debridement, and dressing changes) or standard care only the study measured the VAS related to debridement and overall pain, the analgesic onset and duration of sevoflurane, daily opioid use, ulcer size reduction, quality of life, incidence of tolerance, and adverse events. | Patients who received sevoflurane treatment had lower VAS scores, less opioid use, increased quality of life, and decreased ulcer size at the end of the study period. Sevoflurane was found to induce a fast, intense, and long lasting analgesia, without any evidence of tolerance. Adverse events included mild localized reddening and pruritus (4 out of 10). |
| Imbernon-Moya et al. (38) | Treatment of chronic venous ulcers with topical sevoflurane: A retrospective clinical study | Retrospective review: 30 patients > 65 y who had chronic venous ulcers with pain ≥ 4 on VAS received cleaning with sevoflurane every 2 days for 1 month. | Mean VAS was 8.8 prior to cleanings and 0.8 after the 12th cleaning. Ulcer depth and size also had significant decreases. |
| Imbernon-Moya et al. (39) | Pain, quality of life, and functional capacity with topical sevoflurane application for chronic venous ulcers: A retrospective clinical study. | Retrospective review: 30 patients > 65 y who had chronic venous ulcers with pain > 4 on VAS received cleaning with sevoflurane every 2 days for 1 month. | Latency of analgesic effect after sevoflurane treatment ranged from 2 to7 m and duration ranged from 8 - 18hours. Other important parameters were improved quality of life and functional capacity in patients treated with sevoflurane. Sevoflurane also showed a favorable safety profile with no systemic toxicity and mild local adverse effects such as pruritus, erythema and heat. |
| Imbernon-Moya et al. (40) | Healing of chronic venous ulcer with topical sevoflurane. | Retrospective review: 30 patients > 65 y who had chronic venous ulcers with pain > 4 on VAS received cleaning with sevoflurane every 2 days for 1 month | In addition to the latency, analgesic, quality of life and functional improvements noted above, this publication also notes a decrease in ulcer size and, with a mean ulcer size of 8·4 ± 9·7 cm2 at the beginning of the study and 4·2 ± 5·4 cm2 at the end of the study. |
| Imbernon-Moya et al. (41) | Analgesic and healing effect of topical sevoflurane for chronic venous ulcers | Retrospective review: 30 patients > 65 y who had chronic venous ulcers with pain ≥ 4 on VAS received cleaning with sevoflurane every 2 days for 1 month. | Mean VAS was 8.8 prior to cleanings and 0.8 after the 12th cleaning. Ulcer depth and size also had significant decreases. |
| Martínez-Monsalve et al. (42) | Analgesic effectiveness of topical sevoflurane to perform sharp debridement of painful wounds | Retrospective review: 152 records of patients approved for off label use of sevoflurane as an analgesic in sharp debridement of painful wounds were reviewed. | Baseline pain was 7 on a 0 - 10 numeric rating scale. After irrigation with sevoflurane, median pain level was 2 at 5minutes. Effect lasted a median of 9hrs. |
| Gerónimo-Pardo et al. (43) | Analgesic effect of topical sevoflurane on venous leg ulcer with intractable pain | Case report: Patient with necrotic left external maleolar ulcer, refractory to acetaminophen, metamizol, tramadol, morphine, fentanyl, buprenorphine, pregabal- in, gabapentin, and applications of lidocaine/prilocaine eutectic cream and infusion of epidural ropivacaine. | Original pain was 8/10 and interfering with sleep and application of local treatments. After application of sevoflurane, pain dropped to 4/10 within 2 minutes and lasted for 12 hours. 16 days of treatment resulted in resolution of depth and marked reduction in size of the ulcer. |
| Rueda-Martínez et al. (44) | Topical sevoflurane and healing of a post-operative surgical site superinfected by multi-drug-resistant pseudomonas aeruginosa and susceptible staphylococcus aureus in an immunocompromised patient | Case report: Patient status post liver transplant with pseudomonas infection of incision site who was unable to use antibiotics given renal failure. Incision was subsequently super-infected with staph aureus. | Incision site irrigation with topical sevoflurane resulted in closure and healing of the site likely due to a direct antimicrobial, local analgesic, and direct vasodilator effects. |
| Imbernon-Moya et al. (45) | Application of topical sevoflurane before cleaning painful ulcers | Procedure description | Use of topical sevoflurane as an analgesic is indicated in painful cutaneous lesions refractory to conventional pain control methods. |
Summary of Relevant Literature on the Use of Topical Sevoflurane as an Analgesic
3.1. Chronic Venous Ulcers
Although this space is relatively nascent, there have been randomized controlled studies comparing CVI ulcer pain resolution between patients treated with topical volatiles and controls. One such study compared sevoflurane to halothane and isoflurane at low (2.7 mL, 1 mL, 1.5 mL, respectively) and high (5 mL of sevoflurane vs. water; 2, 4, 6 mL of halothane vs. water) doses. Of topical anesthetics, halothane showed the most potent anesthetic effect (at both low and high doses). Sevoflurane (5 mL) was able to attenuate pain roughly at an equivalent level as 2 mL halothane (32).
Another trial compared 1 - 4 times daily application of 1 mL sevoflurane per 1 cm2 ulcer area (in combination with standard wound care) to standard wound care without sevoflurane. This study reported on several parameters between the two groups. Most notably, when compared with the group receiving standard wound care alone (n = 5), the sevoflurane group (n = 10) had significant (P = 0.001) reductions in the mean ± SD scores for the following categories: debridement-related pain and daily opioid use. Their findings also showed reductions at a lower level of significance in overall pain and ulcer size (26). Although the sample size in this study was small, the marked improvements across the clinically important areas of pain, ulcer healing, and quality of life are encouraging for further studies with increased power.
There have also been a series of publications on a retrospective clinical study done in the space that has reported on a range of parameters from pain resolution, quality of life, and ulcer size and depth. This study evaluated 30 cases of patients with chronic venous ulcers who underwent wound cleaning with topical sevoflurane (1 mL of sevoflurane per 1 cm2 ulcer area, every two days for a month) and compared outcomes with historical results from these same patients who had previously undergone wound cleaning without topical sevoflurane. Of note, patients reported a decreased VAS pain score during cleaning (from an average of 8.8 in prior cleanings without sevoflurane to 0.8 in cleanings with sevoflurane). The patients also reported shorter latency and longer duration of analgesia with sevoflurane and increased quality of life and functional capacity compared with non-sevoflurane wound cleanings (41). Further, the patients treated with sevoflurane also showed improved wound healing after cleaning, with ulcer size and depth decreasing as compared with non-sevoflurane wound cleanings (39). Adverse events from sevoflurane, such as pruritus, erythema, heat, and irritative dermatitis, were mild and self-limited, and no systemic toxicity was reported (38, 40).
Additionally, a retrospective review of 152 medical records of patients that had agreed to be treated using sevoflurane (1 mL/cm2) as an analgesic for sharp debridement of painful wounds showed promising results. The pain was evaluated using a 10-point patient-reported, numerical scale, and median baseline pain across the 152 records was a 7 out of 10. Analgesia was rapid and long-lasting with sevoflurane irrigation with median pain score dropping to 2 out of 10 after sevoflurane irrigation and the median duration of analgesia reported at 9 hours after irrigation (before returning to baseline levels of pain). Importantly, the debridement was completed in 93% of the cases, and adverse effects were mild, with only 34% (n = 52) of patients reporting mild itching after irrigation (42).
There are also case reports that detail the efficacy of topical sevoflurane for CVI ulcer induced pain. One report highlights the case of a 76-year-old woman with a four past medical history of arterial hypertension and atrial fibrillation admitted to the vascular surgery department at Complejo Hospitalario Universitario de Albacete with intense, intractable pain associated with a necrotic left external malleolar ulcer. The patient’s pain was not manageable with several combinations of analgesic agents (acetaminophen, metamizole, tramadol, morphine, fentanyl, buprenorphine, pregabalin, gabapentin, applications of lidocaine/prilocaine eutectic cream, and infusion of epidural ropivacaine) (46-49). Pain control was finally achieved when the ulcer bed was irrigated with 5 ml of liquid sevoflurane. The patient reported a drop in her neonatal abstinence syndrome (NAS) score from 8/10 to 4/10, an onset of analgesic effect within 2 minutes of sevoflurane irrigation, and a duration that lasted 12 hours after application (before her pain relapsed to the pre-treatment score of 8/10). Notably, the patient was able to rest and sleep following sevoflurane application, and a continued daily regimen of sevoflurane irrigation helped achieve ulcer healing within 16 days (43).
Another case studied the application of topical sevoflurane for rescue analgesia in CVI ulcers with refractory pain with direct wound debridement. One patient reported a drop from 10 to 8 on a simple verbal pain scale within 1 minute of sevoflurane irrigation with direct debridement of the wound and return to activities of daily living (ADLs) with full independence within 12 months of sevoflurane irrigation with scheduled wound debridement (42).
3.2. Alternative Uses of Topical Volatile Anesthetics
Topical volatile agents have also shown utility in areas of CVI ulcer treatment beyond pain management. In a 43-year-old male, status-post liver transplant (due to hepatitis C induced cirrhosis), topical sevoflurane rinses were used on a surgical site wound infected initially by multi-drug-resistant Pseudomonas aeruginosa. The patient was initially treated with parenteral colistin, but this had to be discontinued due to deteriorating renal function (44).
Consequently, conservative treatment with daily lavage (non-sevoflurane) and debridement was unsuccessful and led to superinfection with Staphylococcus aureus. The patient was eventually switched over to daily sevoflurane irrigations, which was successful and led to wound healing and closure of the infection site. The authors postulate that the wound healing is attributable to the multiple mechanisms of action of sevoflurane (detailed in an earlier section), including its direct antimicrobial properties, analgesic properties (that improved wound cleaning and dressing), and vasodilatory effects of sevoflurane, which could increase nutrient supply to the wound bed (44).
Topical volatiles has also shown efficacy in attenuating pain from venous cannulation. A comparison of sevoflurane, halothane, and isoflurane at equipotent doses based on MAC (2.7 mL, 1 mL, 1.5 mL, respectively) showed a significant reduction in the incidence and severity of pain (as judged by VAS scores during and following venous cannulation. Out of these, halothane was the most effective at reducing the severity of pain, although sevoflurane and isoflurane also showed some utility (37).
3.3. Safety and Efficacy
The efficacy of topical sevoflurane is measured by the reduction of pain in patients with chronic venous ulcers, in addition to the decrease in area and depth of the ulcer. Repeated irrigation with topical sevoflurane has been shown to significantly reduce the pain score from 9 to 2, reported in visual analog scale, as early as the second day of application (26). In contrast to sevoflurane, regular wound care practices without sevoflurane did not show any decrease in pain scores at the end of a 90-day period with daily wound cleaning and dressing changes. Also, patients undergoing sevoflurane treatment also report higher scores for the Charing Cross Venous Leg Ulcer Questionnaire, which is used to measure the quality of life, and the Barthel index, which is an indication of the functional capacity of the patient (41). The significant reduction in pain during the cleaning and debridement of ulcer after topical sevoflurane application and increase in functionality and quality of life help patients continue the periodic wound-care regimens, which in turn accelerate the healing process.
The administration of analgesic drugs to patients with chronic venous ulcers is a common clinical practice. Along with the reduction of pain after topical sevoflurane treatment, the concurrent intake of other analgesic drugs, including paracetamol, NSAIDs, and metamizole, was also decreased (40). Also, the concentration of morphine sulfate administered to the patients was significantly lower, compared to treatment with just regular wound-care practices (26). Due to the safety issues posed by morphine and similar analgesic agents, the reduction in the morphine sulfate administration makes wound-care and pain management a safer practice with sevoflurane.
The recommended application of topical sevoflurane was reported as irrigating the ulcer wound with 1 ml of liquid sevoflurane per 1 cm2 of the ulcer area while protecting the healthy skin around the ulcer (45). As mentioned earlier, the exact mechanism of action for the analgesic effects of sevoflurane is unknown; however, sevoflurane has been reported to have a short latency time compared to existing analgesic agents. Application of topical sevoflurane to the ulcer at a dose of 1 ml per cm2 of ulcer area was shown to induce analgesic effects in 2 to 7 minutes (45). This, compared to the analgesic onset of lidocaine and prilocaine, which ranges from 30 to 40 minutes, is significantly faster (42, 50).
In addition to the rapid onset, the duration of analgesia was long-lasting with sevoflurane application. A prospective study conducted by Fernandez-Gines et al. report the duration of the analgesic effect of topical sevoflurane as between 8 to 18 hours after applying the recommended dose once (26). The dose-dependency of sevoflurane’s analgesic and healing properties have not been widely studied. However, the subcutaneous administration of similar inhaled anesthetics, including halothane and isoflurane, have shown concentration-dependent analgesic effects (27). Considering the similar molecular structure and anesthetic effects between sevoflurane and other volatile agents, sevoflurane is also expected to induce analgesia in a dose-dependent manner. Overall, the pharmacological characteristics of sevoflurane, including the short onset and extended period of analgesia, make this agent a great candidate for the treatment of chronic venous ulcers and wounds with different etiologies.
3.4. Manufacturing and Production
The applications of sevoflurane and other topical anesthetics described in this paper were all irrigations done with liquid formulations. The formulations were measured using either the minimum alveolar concentration (MAC) of the volatile formulation or mL per cm2 of the ulcer area. The frequency of the rinses differed by study, and details can be found in Table 2.
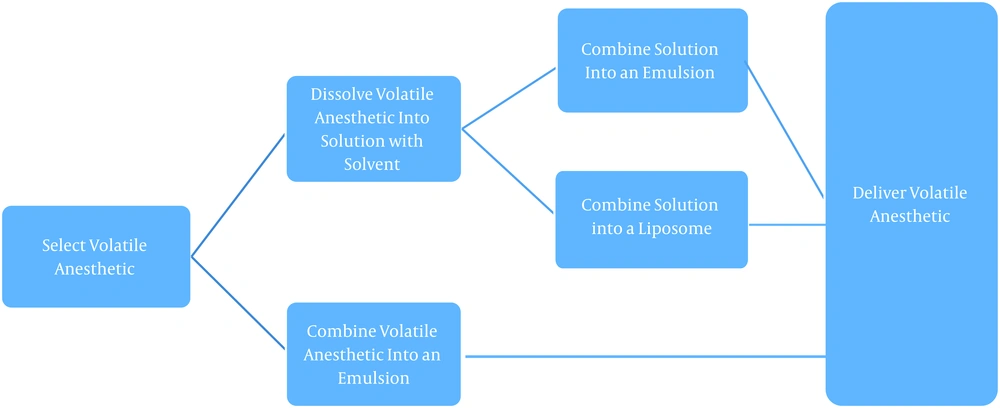
The exact composition of the liquid volatile formulations is mostly proprietary though the overall process is highlighted in Figure 1. There are three predominant versions of the formulation: The volatile anesthetic of choice dissolved in an aqueous-based solution, comprised of a pharmaceutically acceptable extractive solvent (i.e., DMSO or NMP, ranging from 0.1% to 75% of the composition); The volatile agent in an emulsion, and finall The volatile agent in a liposome or microdroplet (51).
Manufacturing outline, volatile anesthetics (51)
The manufacturers of the topical solution postulate that the first of these versions- the aqueous-based solution in an extractive solvent- might hold the most significant promise for clinical use (47). The presence of the extractive solvent provides certain advantages such as improved physical characteristics (improved shelf life, durability, usability, and decreased vaporization or evaporation of the agent), enhanced pharmacological properties (altered pharmacokinetics to provide improved pain relief), and improved usability (reduced volatility resulting in improved ease of handling and safety) (51).
Further details on the various dosages and formulations tested by the manufacturing company can be found in US Patent 2011/0159078 A1 (volatile anesthetic compositions and methods of use).
4. Conclusions
CVI induced venous stasis ulcers are a prevalent condition with a need for advancement in the current treatment paradigm. Current treatment of VSUs is costly, has a poor prognosis, and results in high recurrence, inadequate pain management, and significantly reduced quality of life (QoL). Out of these, the need for better pain management in patients with chronic VSUs is well documented and also correlated with improving both prognosis and quality of life.
Through a mostly unknown mechanism of action, topical volatile anesthetics, such as sevoflurane, have shown a higher degree of pain reduction when compared with traditional analgesic agents such as lidocaine, prilocaine, or oral analgesics (NSAIDs, acetaminophen, metamizole, tramadol, and opioids). As an added benefit, topical volatiles has relatively mild adverse effects and do not have the addictive potential of oral agents such as tramadol and opioids.
The incorporation of volatile anesthetics into VSU treatment plans offers a potential for a paradigm shift in VSU pain management. In addition to improving several parameters of disease burden in individual patients, this shift can also lead to systemic, public health improvements by reducing the burden of opioid addiction and opioid-related adverse effects. Although a body of literature has started to build around the use of sevoflurane and other topical anesthetic agents, the space is still nascent.
In summary, the high potential of these agents in improving a significant burden of disease caused by VSUs warrants more research into their use. Most of the current literature consists of case reports and retrospective analyses, and more robust studies such as randomized clinical trials can go far in elucidating the proper role of volatile anesthetics in VSU treatment plans. Given the numerous advantages that topical volatiles offer compared to conventional analgesics, further study in this space is highly recommended and needed.