1. Introduction
Neuropathic pain can be caused by several pathologies affecting the nervous system. Peripheral
neuralgias may be related to nerve entrapment, traumatic or iatrogenic events, and may alsoaccompany many other diseases. Very often, neuropathic pain syndromes of peripheral origin are resistant to conventional medical treatments. Peripheral nerve stimulation (PNS) is effective in treating many of these neuralgic syndromes. PNS is increasingly used in chronic neuropathic pain that can be attributable to a specific nerve, a specific nerve, with a corresponding distribution (1-4).
2. Case Presentation
We treated two patients suffering from chronic neuropathic pain of peripheral origin (5). Toconfirm the diagnosis, an ultrasound-guided (6) diagnostic nerve block was performed in each patient to evaluate the effectiveness of pain relief on the target nerve with local anesthetic (Lidocaine Hydrochloride 2% 1 cc) using a Quincke spinal needle 22G 90 mm. For further confirmation, a second ultrasound-guided diagnostic block was repeated after one week. Patients who responded to both nerve blocks were treated with the implantation of a Bioness (Valencia CA, USA) StimRouter® peripheral nerve stimulation (PNS) system comprised of a fully implanted,15 cm permanent lead, a “wearable” External Pulse Transmitter that powers the lead through the skin, and a Patent Programmer that resembles a small remote control the patient uses to turn the device on/off and up/down. Patients were provided written informed consent before the diagnostic blocks and the PNS procedure. We assessed the outcome by measuring the pain with the Numerical Rating Scale (NRS) and SF-12 questionnaire for assessing the impact of health on an individual's everyday life. The Physical Score (PCS-12) and the Mental Score (MCS-12) were evaluated with the SF-12. The questionnaires were administered before and after the implant procedure and were also evaluated with follow-up visits at 1 - 3 - 6 - 12 months. PNS was performed without a trial stimulation phase. In each patient, the device was programmed/activated the day after the implant procedure. The stimulation parameters were chosen based on paresthetic coverage and patient comfort. At least, two stimulation programs were stored on the external pulse transmitter that the patient could manage. The procedure has been performed on an inpatient basis or day-surgery. The average duration of each procedure was 30 minutes.
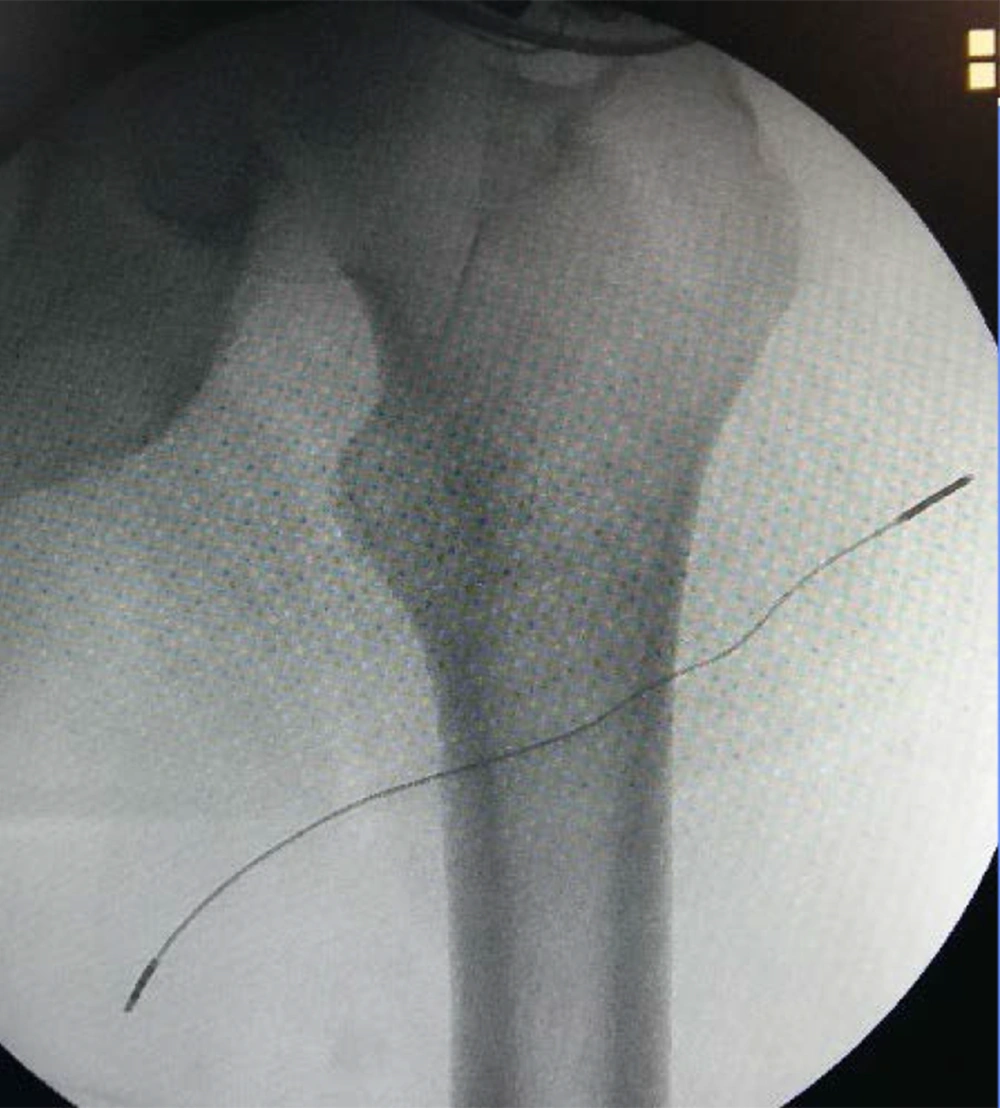
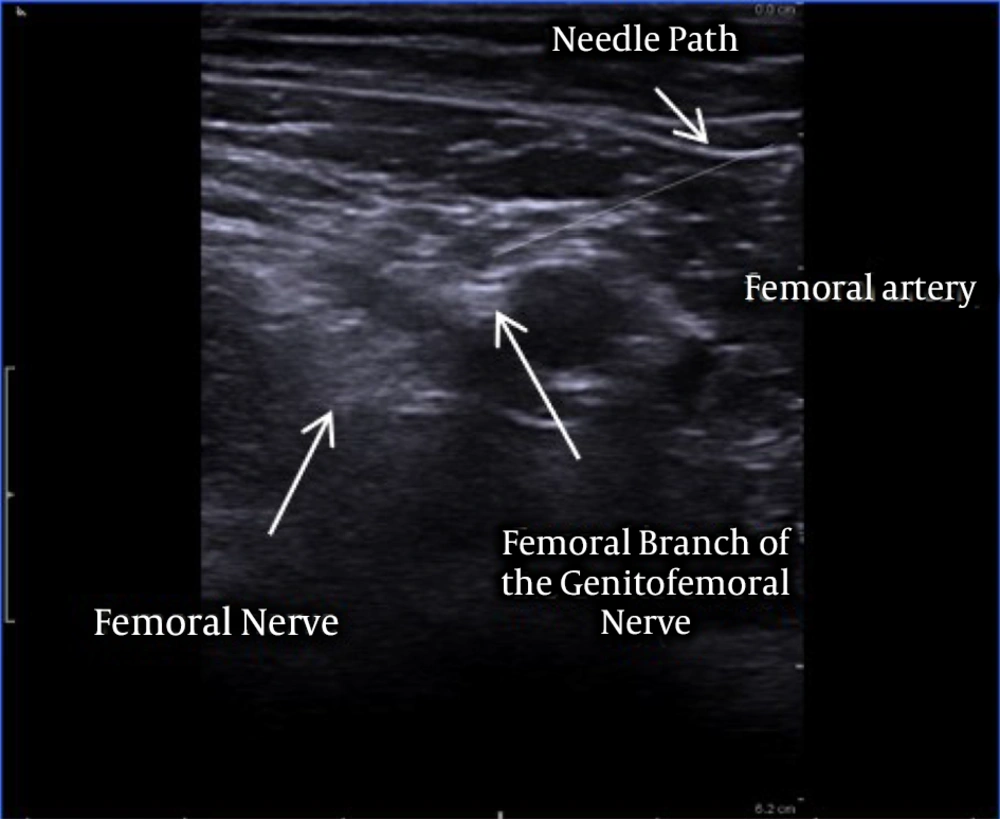
First patient: A 77-year-old male patient suffered from severe neuropathic chronic pain, with intense burning and shooting pain in the medial part of the thigh and the ipsilateral scrotum, unresponsive to all conservative treatments. Although he underwent numerous medical examinations, no reason had been identified for his chronic neuropathic pain syndrome. The patient has no major comorbidities. The main component of this neuropathic chronic pain syndrome was presumably due to the femoral branch of the left genitofemoral nerve, considering the complete pain relief obtained with the diagnostic block of the femoral branch. The patient’s pain intensity was described before the implantation as 9. SF-12 preoperative values were PCS-12: 27.2 and MCS-12: 29.3. The patient was then implanted in January 2017 with Bioness StimRouter® implantable PNS system. Under ultrasound guidance (Toshiba Aplio 300 Ultrasound Machine) using a linear probe 12 MHz, the lead was placed, under the tensor fasciae latae superficially and perpendicular to the left femoral branch of the genitofemoral nerve (Figures 1 and 2). Stimulation parameters used: burst symmetric wave, pulse width 120 ms, frequency 100 Hz, amplitude 1 mA.
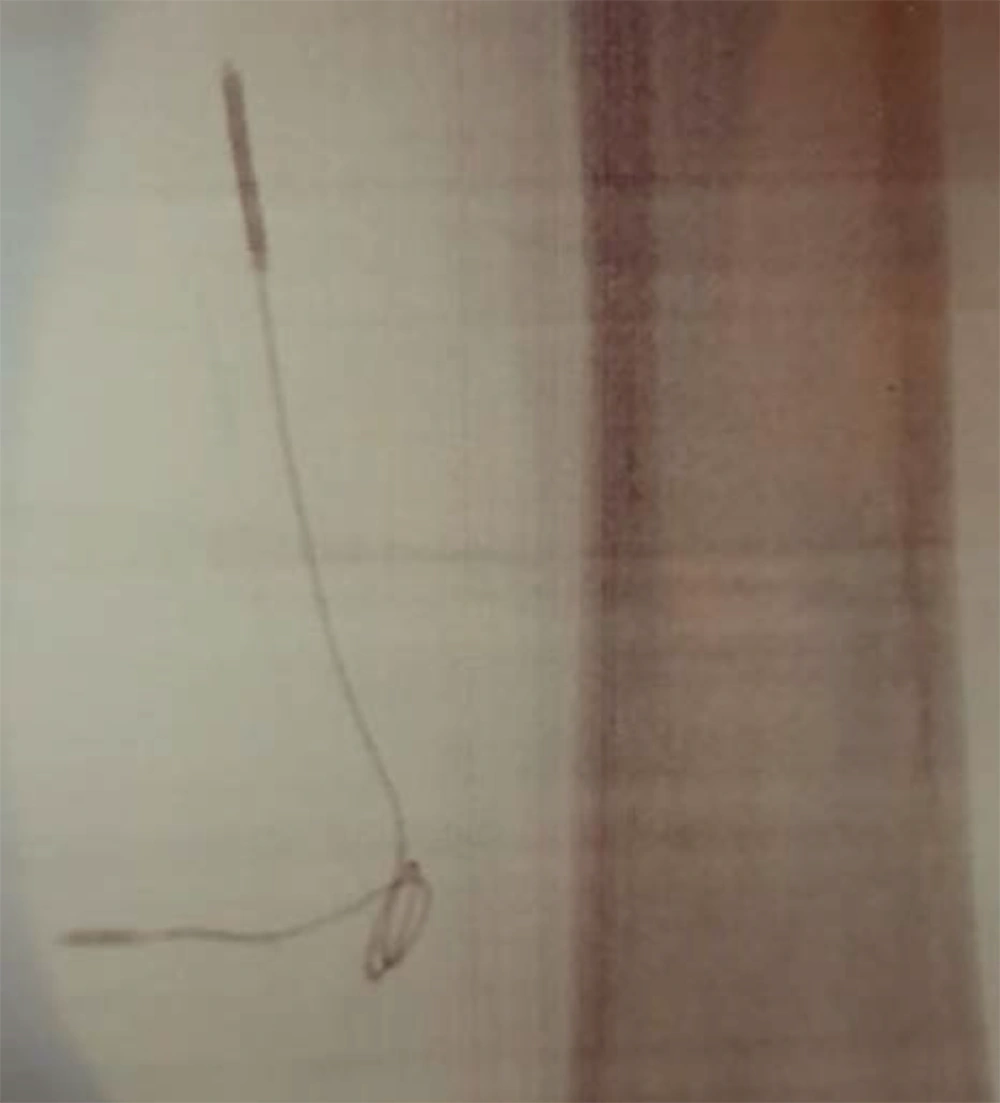
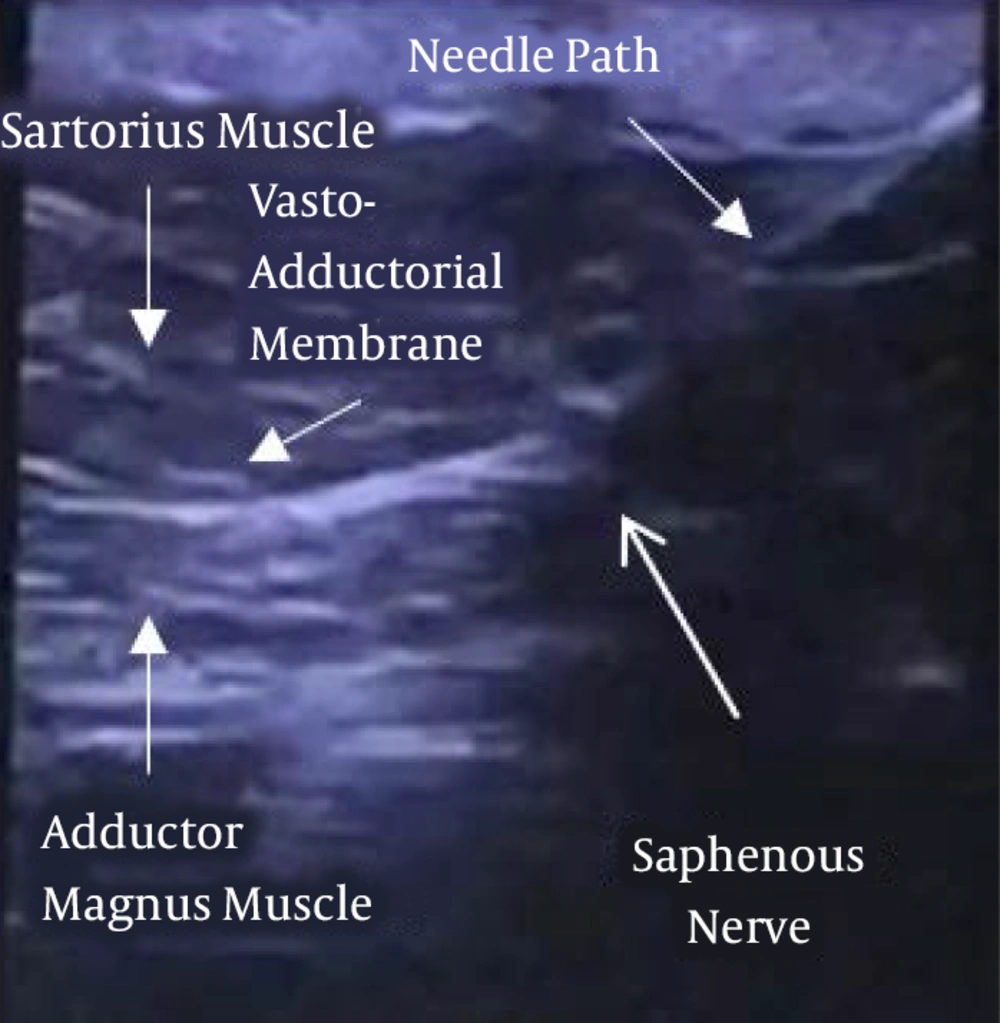
Second patient: A 69-year-old female patient, without major comorbidities, suffered from persistent neuropathic pain in the anteromedial aspect of the left knee, presumably due to the infrapatellar branch of the saphenous nerve (IPSN) neuralgia. The patient was subject to many surgical interventions since 2012 without any benefit. The patient’s pain intensity was described before the implantation as 9. SF-12 preoperative values were PCS-12: 28.9 and MCS-12: 27.3. The patient was implanted in February 2019 with Bioness StimRouter® PNS system. The lead was placed, under ultrasound guidance (Toshiba Aplio 300 Ultrasound Machine), with the patient in the supine position with external rotation of the limb to be treated. A linear probe 12 MHz was used to identify the saphenous nerve in the lower third of the thigh. The lead was then positioned with a latero-medial in-plane approach perpendicular to the left saphenous nerve. The part of the lead with the receiver was tunneled into the upper portion of the thigh (Figures 3 and 4). Stimulation parameters were as follows: cyclic symmetrical wave 40 sec ON 10 sec OFF, pulse width 200 ms, frequency 100 Hz, amplitude 1.1 mA.
3. Discussion
In the first patient, pain relief was significant from the beginning, and a value of NRS 3 persisted in all follow-up visits, as well as the SF-12, with values of PCS-12: 39.6 and MCS-12: 53.1. The second patient had outstanding pain relief also from the very beginning. The NRS was reduced from 9 to 2 and persisted in all follow-up visits. SF-12 postoperativevalues were PCS-12: 47.4 and MCS-12: 49.7. The values of the SF-12 remained stable over time in both patients. Additionally, the first patient reported a decreased opioid intake of 50% and neuropathic pain medications as well. The second patient reported the discontinuance of opioid intake and all the other pain medications previously taken. Both patients would recommend this therapy to other patients suffering from chronic neuropathic pain of peripheral origin. There were no adverse events related to the procedure. PNS performed with the StimRouter® system, implanted percutaneously under ultrasound guidance is safe and effective for patients. PNS was shown to have a great impact on the patient’s quality of life. The significant reduction or interruption of drug intake is crucial, especially for opioids (7). The minimally invasive procedure with a surgical incision of less than 1 cm was performed while the patient was awake under local anesthesia only. No complications were caused using the patient’s feedback during stimulation and placement. The PNS procedure described allows for the treatment of patients who will not be candidates for more invasive procedures under general anesthesia because of age or other comorbidities.