1. Background
Labor pain is one of the most painful experiences in a women's life. Neuraxial analgesia techniques are considered the most effective methods to provide pain relief during labor. epidural analgesia is the most common technique for pain relief during labor (1). Anozie et al. evaluated the effects of epidural analgesia in a sample of Nigerian obstetricians and mentioned high costs and lack of sufficient skills as reasons for not using the epidural technique (2). Meanwhile, some clinicians prefer spinal analgesia to epidural analgesia. This method contains using small doses of local anesthetic, mainly because it can spread directly in the spinal fluid. On the other hand, while performing in the same way, spinal analgesia requires a thinner needle to make a spinal block, which means a tiny hole in the dura. Moreover, a spinal block technique can take a shorter time than an epidural block (3). Some studies mentioned that spinal analgesia not only can be applied more easily but also is faster, less expensive, and more effective than epidural analgesia (4-11). Administration of opioid drugs in neuraxial blocks, which do not affect the sympathetic activity, along with local anesthetics, is a common technique to avoid negative consequences such as hypotension in these techniques (12-16). However, the local anesthetic dose that can safely provide effective, long-lasting labor analgesia without motor block must be determined. Therefore following a prospective clinical trial design, the current study aimed to compare the spinal analgesia with combined local anesthetics and narcotics versus intermittent epidural bolus analgesia to manage labor pain.
The primary outcome was comparing the visual analog scale of parturient after regional analgesia and analgesia duration and secondary outcomes included the labor duration, mode of delivery, Apgar score of neonates, anesthetic complications, and maternal satisfaction.
2. Methods
This single-blind, prospective, randomized, clinical trial study was conducted in Arash Women's Hospital from February 2019 to October 2020. The research was registered in the Research Ethics Committee of Tehran University of Medical Sciences. Written informed consent was obtained from all patients. A total of 128 nulliparous or multiparous parturient women who requested analgesia during labor were enrolled. Inclusion criteria were being aged 18 to 35 years, ASA physical status I or II, at term, singleton, with cervical dilation between 5 - 6 cm, and availability of normal fetal heart rate (FHR) tracings. Patients with sedative drug abuse, opium addiction, previous spinal or epidural analgesia failure, presence of underlying diseases like hypertension, diabetes, coagulopathy, preeclampsia, and epilepsy, and other central nervous system disorders, and those with INR > 1.3 or platelets count < 100,000 were excluded from the study. The randomized block design was applied for the random allocation of participants to the study groups. Participants were then randomly allocated to one of the spinal (n = 64) or the epidural groups (n = 64). Data were collected by a trained nurse.
2.1. Intervention
For all participants, the demographic and baseline data were collected using a questionnaire, which included sections about age, height, weight, nulliparous or multipara, cervical dilation at the time of performing labor analgesia, and pain intensity before the procedure. Before providing the intervention, all patients received 500 mL Ringer solution. Baseline values of pulse rate (PR), respiratory rate (RR), hemoglobin oxygen saturation (SpO2), and mean arterial pressure (MAP) were recorded. All procedures were performed in the sitting position under aseptic conditions by an expert anesthesiologist. Patients in the spinal group received spinal block using a 25 gauge Quincke needle. The puncture was performed between L4 - L5 intervertebral space, and after a successful dural puncture, 0.5 mL (2.5 mg) hyperbaric bupivacaine 0.5% with 50 μg fentanyl was injected. If the pain intensity was ≥ 5 and the patient requested additional analgesia, the physician was allowed to inject 50 mg of meperidine intramuscularly. For those in the epidural group, using a loss of resistance technique, a 17 gauge Tuohy epidural needle was performed in L4 - L5 space, and an epidural catheter was introduced. After confirming the catheter's safe position, 16 mL bupivacaine (0.125%) + 50 μg fentanyl was injected into the epidural space. Regarding the intensity of pain score ≥ 5 and patient request, 5 - 10 mL of the same solution was administered via the epidural catheter by the anesthesiologist. All patients were returned in the supine position with a mild head-up position and left uterine displacement for the first 15 min; then, they were allowed to have any desired position or walking.
2.2. Outcomes Measurement
After administration of the anesthetic solution and recording the vital signs, the severity of pain was recorded using the visual analog scale (VAS) with a 10-point scale, ranging from zero (no pain) to 10 (intolerable pain). The pain was measured before performing the regional anesthesia and in the following, every 15 minutes until delivery. The duration of analgesia was determined as the time after neuraxial block until the VAS score rises ≥ 5.
The FHR was evaluated using continuous electronic FHR monitoring throughout labor, and deceleration of FHR was recorded. The duration of the first and second stages of the labor, the cesarean section (C-section) rate, instrumental vaginal delivery, and incidence of postpartum hemorrhage were recorded for both groups. Apgar scores of all newborns were recorded at 1 and 5 minutes after birth. Patients' satisfaction was evaluated by a four-level scoring system as excellent, good, moderate, and low. Patients were monitored for any sensory or motor complication as well as the presence of any side effects such as hypotension (as a 20% decrease from baseline), SpO2 decline, Pruritus, nausea or vomiting, and urinary retention. The sample size was estimated as 6 subjects per each group, based on previous studies (1.97 ± 1.14 in the spinal group and 2.63 ± 1.49 in the epidural group) and with a statistical test power of 80% and type one error of 0.05. The study is approved by the Ethics Committee of the Tehran University of Medical Sciences (IR.TUMS.MEDICINE.REC.1398.077). In addition, it was registered at IRCT (code: IRCT20121006011020N14) (https://en.irct.ir/trial/48305).
2.3. Statistical Analysis
Data were analyzed using SPSS version 20. Qualitative variables are described using frequency percent, and quantitative variables are described using mean ± standard deviation. Data were compared using Student's t-test for quantitative variables. The chi-square test was used to compare the categorical variables between the two groups. We considered mean total VAS scores as a binary outcome. VAS scores < 5 were considered as successful analgesia. The outcomes were adjusted for maternal age, weight, height, gestational age, fetal weight, gravidity, cervical dilation at the time of performing labor analgesia, and baseline pain score using univariate and multivariate logistic regression models. Statistical significance was considered when P-value < 0.05.
3. Results
We enrolled 128 parturient in the active phase of labor. Sixty-four women were assigned to receive epidural analgesia and 64 parturient to receive spinal analgesia. All 128 parturient women enrolled completed the study. No technical difficulty was found in any patient (Figure 1). Demographic data and baseline characteristics are illustrated in Table 1. There was no statistically significant difference between the two groups concerning age, weight, height, gestational age, gravidity, and cervical dilation at the time of performing labor analgesia (P ≥ 0.05) (Table 1).
| Parameters | Spinal Group (n = 64) | Epidural Group (n = 64) | P-Value b |
|---|---|---|---|
| Age (y) | 27.7 ± 4.7 | 26.3 ± 5.2 | 0.12 c |
| Weight (kg) | 65.53 ± 11.17 | 65.53 ± 11.98 | 0.59 |
| Height (cm) | 160.45 ± 5.3 | 160.73 ± 6.43 | 0.79 |
| Gestation age (week) | 39.4 ± 1.08 | 39.1 ± 1.1 | 0.16 |
| Primi gravida, No. (%) | 32 (50) | 31 (48.4) | 0.86 |
| Cervical dilatation before analgesia (cm) | 5.40 ± 0.5 | 5.4 ± 0.5 | 0.86 |
a Values are expressed as mean ± SD.
b P-value was obtained from independent sample t-test.
c P < 0.05 considered statistically significant.
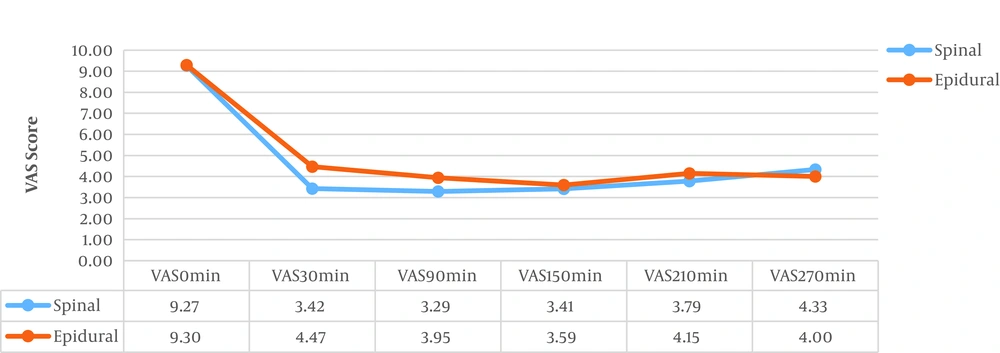
Duration of the labor was longer in the epidural group than the spinal group, but this difference was not statistically significant (Table 2). Temporary fetal bradycardia in the first 30 minutes after neuraxial analgesia was found in 8 (12.5%) patients in the spinal group versus 7 (10.9%) cases in the epidural group, which were treated using the administration of 500 mL of crystalloids and oxygenation and keeping the patients at the left lateral position (Table 2). The mean VAS score before analgesia was similar between the two groups. After performing analgesia, the VAS score was significantly reduced in the spinal group compared to the epidural group 30 (P = 0.0001) and 90 minutes (P = 0.01) after analgesia. However, there was no significant difference between the spinal and the epidural groups concerning VAS scores at 150, 210, and 270 min (Table 3 and Figure 1). The mean duration of analgesia was significantly higher in the spinal group than the epidural group (224.8 ± 18.9 min in the spinal group vs. 158.9 ± 37.2 min in the epidural group; P = 0.0001) (Table 3 and Figure 2).
| Parameters | Spinal Group (n = 64) | Epidural Group (n = 64) | P-Value |
|---|---|---|---|
| Duration of labor (min) | 107.18 ± 61.642 | 117.46 ± 88.84 | 0.459 c |
| Duration of stage 1 (min) | 83.63 ± 53.853 | 93.1 ± 82.64 | 0.45 c |
| Duration of stage 2 (min) | 23.94 ± 21.57 | 28.27 ± 29.37 | 0.41 c |
| Cesarean section | 12 (18.8) | 13 (20.3) | 0.82 d |
| Side effects (proritus) | 8 (12.5) | 0 (0) | 0.003 d |
| Postpartum hemorrhage | 3 (4.7) | 5 (7.8) | 0.46 d |
| Fetal bradycardia e | 8 (12.5) | 7 (10.9) | 0.78 d |
| Apgar scores at 1 min | 8.9 ± 1.1 | 8.4 ± 1.3 | 0.06 c |
| Apgar scores at 5 min | 9.7 ± 0.65 | 9.6 ± 0.87 | 0.46 c |
a Values are expressed as mean ± SD and No. (%).
b P significant level < 0.05.
c P-value was obtained from Independent sample t-test.
d P-value calculated by chi-square test.
e Fetal bradycardia until 30 min after analgesia.
| Parameters | Spinal Group (n = 64) | Epidural Group (n = 64) | P-Value |
|---|---|---|---|
| VAS 0 min | 9.27 ± 0.542 | 9.30 ± 0.525 | 0.74 c |
| VAS 30 min | 3.42 ± 0.94 | 4.47 ± 1.84 | 0.0001 c |
| VAS 90 min | 3.29 ± 0.871 | 3.95 ± 1.34 | 0.002 c |
| VAS 150 min | 3.41 ± 0.70 | 3.59 ± 1.37 | 0.49 c |
| VAS 210 min | 3.79 ± 0.70 | 4.15 ± 0.99 | 0.27 c |
| VAS 270 min | 4.33 ± 0.58 | 4.00 ± 0 | 0.49 c |
| Duration of analgesia (min) | 224.84 ± 18.94 | 158.91 ± 37.21 | 0.0001 c |
| Patient need to rescue dosage | 3 (4.7) | 18 (28.12) | 0.008 d |
| Need to multiple rescue dosage | 0 (0) | 5 (7.8) | 0.058 e |
| Patients’ satisfaction as good and excellent | 61 (95.3) | 49 (76.6) | 0.002 d |
a Values are expressed as mean ± SD and No. (%).
b P significant level < 0.05.
c P-value was obtained from Independent sample t-test
d P-value calculated by chi-square test.
e Fisher exact test.
After adjusting univariate and multivariate logistic regression models, no significant change was observed in the results. In the spinal group, successful analgesia remained stable at 61 (95.31%) women for the entire labor duration. However, 3 (4.69%) women had a VAS score of < 5 or 6 after 180 min. Whereas in the epidural group, 18 women (28.12%) needed a rescue dosage of analgesic drug injection via the catheter, who five of them required two rescue drug injections (Table 3). The number of patients with a good and excellent analgesic quality score was higher in the spinal group [n = 61 (95.3%)] compared to the epidural group [n = 49 (76.6%); (P = 0.002)] (Table 3). Pruritus was more common in the spinal group than the epidural group (P = 0.003). However, the severity of itching was mild among the patients (Table 2). Also, there was no statistically significant difference between the groups regarding C-section rate and instrumental vaginal delivery.
4. Discussion
Our study focused on the safety and efficacy of single-dose spinal analgesia compared to bolus intermittent epidural analgesia during labor. Concomitant with our study, Mazul-SunkoBranka (17) reported a slower onset of analgesia for epidural technique regarding spinal anesthesia. Elkhateeb and Hamdy (18), in a study on 120 women in the active phase of labor, administered a combination of fentanyl (25 mg) and bupivacaine intrathecally (2.5 mg) for participants in the spinal group. Those in the epidural group received 20 mg of bupivacaine (0.5%) plus 50 mg of fentanyl by an epidural catheter. They found that the onset of sensory analgesia (detected by the cold test) was earlier, and the duration of sensory analgesia was longer in the spinal group compared to the epidural group. The duration of sensory block was longer (123.21 ± 8.06 min) in the spinal group than the epidural group (103.0 ± 12.62 min), which is consistent with the findings of the present study, despite the difference in the dose of the local anesthetic drugs. The analgesic effect of intrathecal fentanyl (25 μg) for labor lasted for 60 - 90 minutes. Above this dose, the duration of action was increased. Palmer et al. (19) used 5 - 45 mg of intrathecal fentanyl as part of a combined spinal-epidural technique. Synergy was noted between fentanyl and bupivacaine; thus, we hypothesized that increasing the fentanyl dose to 50 μg with 2.5 mg bupivacaine could prolong the duration of spinal analgesia. Therefore, according to our pilot study, this combined dose can be used at an early stage in appropriately selected parturients without any potential side effects.
Krzysztof and Susilo Chandra (20) used single-dose spinal analgesia to manage obstetric pain in order to assess maternal satisfaction. They reported that a single dose of spinal anesthesia with bupivacaine 2.5 mg plus morphine 0.25 mg and clonidine 45 μg is a good technique for labor analgesia. In addition, they noted that it can cause a good duration of pain relief, a high level of maternal satisfaction, with a very few side effects; Hence, it can be concluded that this is a highly cost-effective and safe method for routine obstetric labor analgesia, which is in line with the findings of the present study. Tarek AbdElBarr et al. (21) compared the efficacy of single-dose spinal analgesia with epidural analgesia during labor. In their study, the women with labor pain were divided into two groups (each with an equal number of subjects). Those in the spinal group received 3.75 mg of hyperbaric bupivacaine with 25 mg of fentanyl. Meanwhile, for those in the epidural group, 20 mg of bupivacaine and 50 mg of fentanyl were administrated. Concomitant with our study, the duration of sensory block in their study was longer (120.4 ± 15.6 vs. 103.2 ± 18.3 min, P < 0.001) in the spinal group compared to the epidural group. They concluded that a single dose of spinal analgesia is a good alternative compared to the epidural route.
Collis (22) found that a single shot spinal technique with the bupivacaine 2.5 mg added to fentanyl 25 μg intrathecally lead to effective analgesia, which continued at least for 90 minutes, while, in the present study, the duration of analgesia was longer than their study. Patrica Fontaine et al. (23) compared the efficacy of spinal and epidural analgesia for labor pain. For those in the spinal group, 0.25 mg of morphine and 25 to 35 mg of fentanyl were injected into the subarachnoid space. In the epidural group, 8 to 10 mL bolus of 0.25% bupivacaine with 50 μg fentanyl were administered. Women receiving epidural analgesia had significantly lower pain scores in the overall postpartum evaluation. The median duration of effective pain relief ranged from 60 to 120 minutes for those who received spinal analgesia. Contrary to the findings of the present study, we found a longer duration of pain relief among those who received spinal analgesia than those in the epidural group, which can be attributed to the combined use of fentanyl and bupivacaine in the present study for those who received the spinal method. Minty RG et al. (24) conducted a meta-analysis to review the literature on obstetric analgesia and pain measurement and concluded that single-dose spinal anesthesia is a useful alternative method to perform epidural analgesia for appropriately selected patients.
4.1. Conclusion
According to the findings, single-dose spinal analgesia, compared to epidural analgesia, is a safe, fast, and efficient technique for labor analgesia, which can be easily performed. In addition, it provides a high satisfaction level in the parturient.