1. Background
Postoperative pain is an important cause of increased morbidity, patient discomfort, and prolonged hospital stay (1). To date, various methods have been used to reduce postoperative pain. To attenuate postoperative pain, the tendency to use regional blocks over systemic analgesics is increasing. One of these blocks to reduce post abdominal surgery pain is ultrasound-guided transversus abdominis plane (TAP) block (2).
TAP block was first introduced about a decade ago by Rafi et al. (3). They performed this block using the Petit triangle landmark and the double-pop method. Over time, while ultrasound was introduced to regional block, it was carried out under the guidance of ultrasound (4). The use of TAP blocks in extensive abdominal surgery has resulted in shorter hospital stays lower analgesic use in the postoperative period, fewer pain scores, and reduced nausea and vomiting (5).
Although the type, volume, and concentration of the local anesthetic used in TAP block vary in different studies, bupivacaine, ropivacaine, and levobupivacaine have been used more extensively (6).
Various studies on the TAP block have not mentioned a particular advantage for the drug infusion method compared with the single-dose method (7). However, the continuous infusion method seems to be more practical, though it cannot be used in an outpatient setting. On the other hand, a single-shot TAP block has a short analgesic duration, which is one of the drawbacks of this method. To overcome this problem and for improvement of single-dose block and prolongation of analgesic effects, various supplements, such as narcotics, clonidine, midazolam, ketamine, magnesium sulfate, and dexmedetomidine have been used (8-10).
Dexmedetomidine is an alpha-2 agonist that has been approved as a venous sedative and an adjuvant for pain relief (8). In previous studies, the addition of dexmedetomidine to bupivacaine in the TAP block has prolonged the effect of this block (6-12). So far, the optimal dose of dexmedetomidine for the TAP block has not been determined.
2. Objectives
This double-blind, randomized, controlled trial was designed to compare three different doses of dexmedetomidine in the TAP block in patients undergoing open inguinal herniorrhaphy for determination of the appropriate dose.
3. Methods
Ethics approval of the Research Council of University was received on March 16, 2017. The initial design of the study was recorded on the clinical trial registry site. The study was conducted over 14 months (from September 2018).
Patients who met the following inclusion criteria were enrolled in this study: (1) the age of 18 - 80 years; (2) anesthesia class 1 or 2 (according to the American Society of Anesthesiologists (ASA) classification; (3) undergoing inguinal herniorrhaphy by spinal anesthesia; (4) ability to understand and rate their pain on visual analog scale (VAS); and (5) ability to understand and signing the written informed consent. The following conditions resulted in exclusion from the study: (1) history of drug abuse; (2) presence of coagulopathy; (3) intolerance of spinal anesthesia and eventual conversion to general anesthesia for the rest of the surgery; (4) history of allergy with bupivacaine or dexmedetomidine use; (5) presence of pain in the area before the surgery; (6) history of psychiatric diseases; (7) history of renal or liver failure; and (8) history of heart disease (ischemic, valvular, heart block).
Following obtaining informed consent, the patients were assigned randomly to three groups using blocked randomization and a computer random number generator. The process of participant enrollment and assigning them to the intervention groups were done by a nurse. In all three groups, the base of the TAP block contained 20 mL of bupivacaine 0.125%, which was supplemented with 0.5, 1, or 1.5 μg/kg of dexmedetomidine and administrated to the groups as low (L), medium (M), or high (H) dose, respectively. Neither the participants nor the experimenters knew who is receiving a particular dose.
The data were collected using a pre-designed checklist and completed at the time of arrival to the operating room to the end of discharge from the hospital. In the operating room, patients were divided into three groups based on random blocking.
All patients received 10 mL/kg of normal saline before induction of anesthesia and underwent monitored by a pulse oximeter, and their blood pressure and electrocardiogram were checked (Saadat Iran Company). The baseline blood pressure and heart rate were recorded. The anesthesia of all patients was similar and included spinal block in sitting position using 15 mg hyperbaric bupivacaine (Marcaine, Spinal Heavy 0.5% AstraZeneca, Sweden). The block was made using needles No. 25 to 27 (Quincke B Braun Germany) and through the intervertebral space L3 - L4 or L4 - L5. After placing the patient in a supine position, oxygen with a face mask and a flow of 6 liters per minute was established.
Side effects, including headache, nausea and vomiting, hypotension, bradycardia, and total spinal block, were recorded in the checklist. The following countermeasures were decided for the management of side effects/complications: (1) bradycardia (heart rate less than 50 beats per minute), 0.7 mg atropine; (2) hypotension (blood pressure reduction of more than 20% baseline), liquid injection in the first stage and in case of no response or a severe drop in blood pressure, ephedrine 5 - 10 mg; (3) nausea and vomiting, ondansetron 1 mg intravenously.
During surgery, the cardiovascular state was measured and recorded by an anesthesiologist (every 5 minutes for the first 20 minutes and then every 15 minutes until recovery).
At the end of the surgery, TAP block was performed by a method similar to Hebbard et al. (4). After sanitizing the upper area of the iliac crest on the operation side with povidone-iodine 10%, and under sterile conditions, the linear probe (linear R) ultrasound was placed in a transverse position on the anterolateral plane of the abdomen in the space between the iliac crest and below the ribs. The fascia between the internal oblique and the transversus abdominis muscles was identified. The pajunk 16 needle was placed under ultrasound guidance in this area, and the drugs were injected after negative aspiration. Care was taken during the injection to ensure that the drugs were properly distributed in the empty and black space of TAP and the kayak sign was formed correctly. All TAP blocks were done by a senior resident.
The primary outcome of the study was pain at rest and coughing, which was measured by the VAS. The pain was measured using a horizontal 10-centimeter VAS that was orientated from the right (no pain) to the left (the most intense pain imaginable) and was recorded at 0, 2, 4, 8, 12, and 24 hours postoperatively. The patient's level of sedation was the secondary outcome, and checked through the four-point modified Ramsay scale and recorded in the checklist immediately after the block and at 2, 4, 8, 12, and 24 hours postoperatively. Patients received a modified Ramsay score of 1 for wholly conscious and attentive, 2 for sleepy but collaborator, 3 for sleepy and little obey in response to commands and sleepy again, and 4 for the sleepy and small response to painful stimulation or loud noise. When the patient first complained of pain, had a VAS score above 5, or requested analgesia, 1000 mg intravenous (IV) injection of acetaminophen was used. During 24 hours, whenever the patient had pain, 1000 mg of IV acetaminophen was injected. The injection intervals were at least 6 hours. In the case of unresponsiveness of pain to acetaminophen, 2 mg of IV morphine sulfate was injected into the patient as a rescue drug. The amount of acetaminophen and morphine sulfate in 24 hours was considered as the amount of analgesic required for pain relief and was recorded.
Patient satisfaction of the block was measured using a five-point scale: (1) 1 for completely unsatisfied, (2) 2 for somewhat unsatisfied, (3) 3 for neither satisfied nor unsatisfied, (4) 4 for slightly satisfied, and (5) 5 for completely satisfied.
3.1. Statistical Analysis
Statistical analysis was done using IBM SPSS version 26, and descriptive statistics, such as mean, standard deviation, frequency, and adjusted frequencies, were reported. Depending on the type of the predictor, Pearson’s correlation, chi-square test, and ANOVA were used to calculate the test statistics and report the P-values. The main assumption of conducting one-way ANOVA, including normality of data and homogeneity of variances, were checked via the Kolmogorov- Smirnov and Levene’s test, respectively. In this study, the P-values less than 0.05 were considered statistically significant.
4. Results
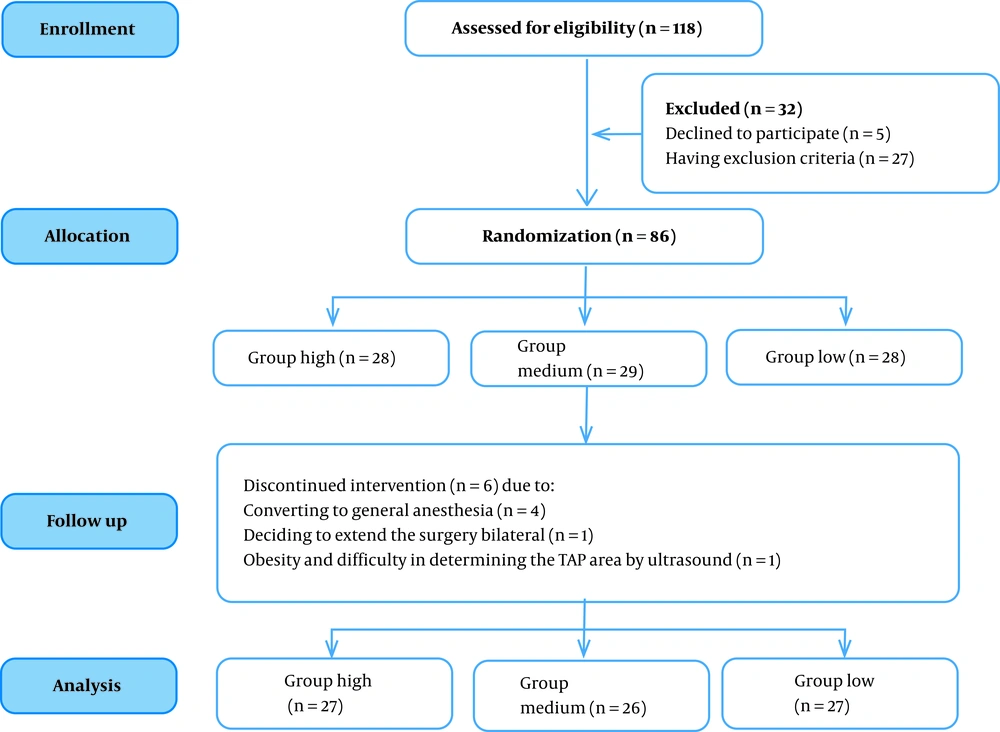
A total of 118 patients were screened for potential inclusion, of which 86 patients were recruited and randomized. Four patients were withdrawn for converting to general anesthesia, and one patient was excluded because of deciding to extend the surgery bilateral, and one patient was excluded because of morbid obesity and difficulty in determining the TAP area by ultrasound. Thus, the final numbers of participants were 80 cases (Figure 1).
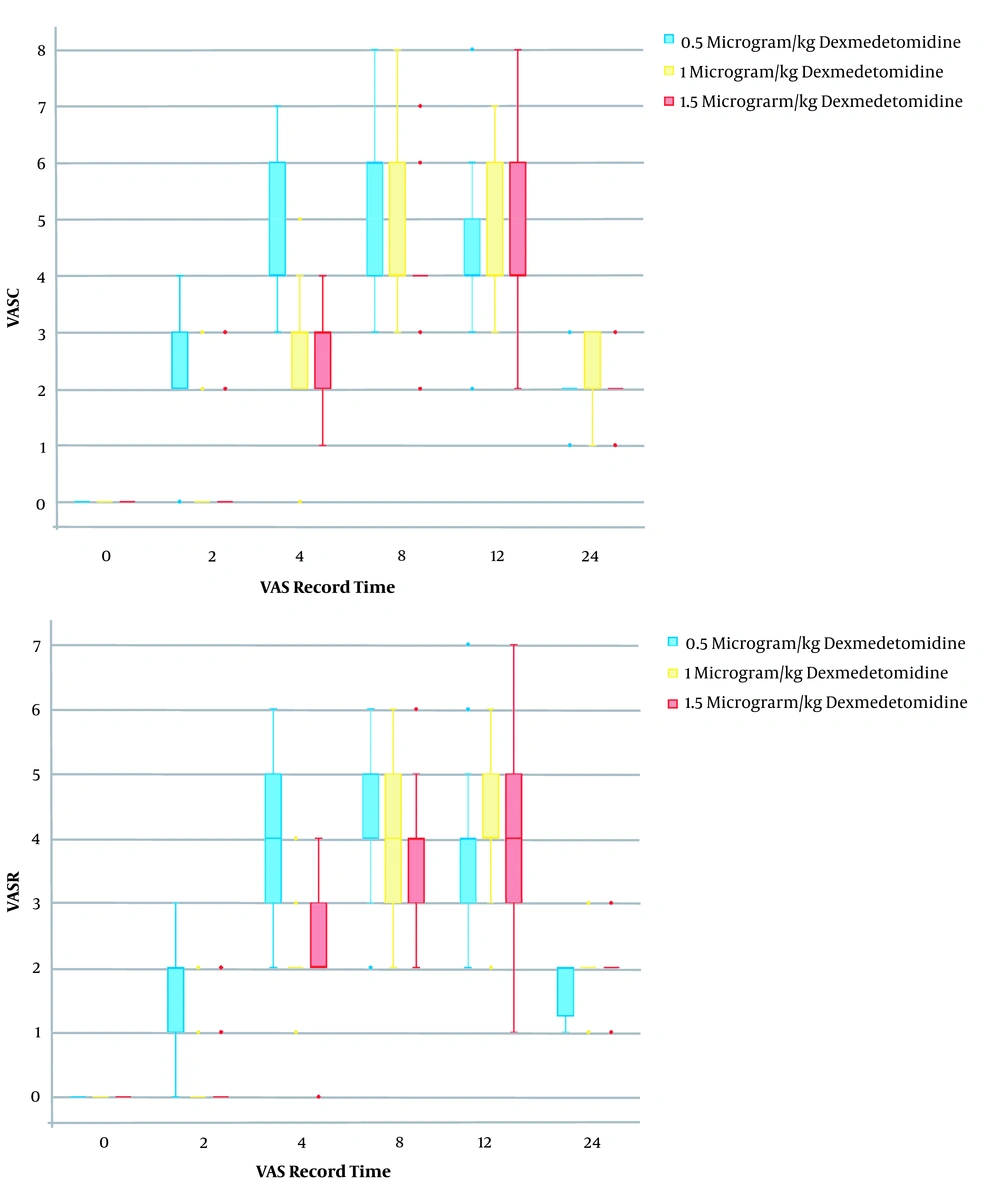
There was no significant difference in age, body mass index (BMI), gender, operation time, anesthesia duration, mean arterial pressure (MAP), heart rate, and complications after spinal anesthesia and TAP block between the three groups (P > 0.05) (Table 1). Significant differences were seen in pain VAS score among the three groups. It was lower in the M and H groups than the L group at 2, 4, and 8 hours postoperatively, at both rest and cough states (Table 1 and Figure 2).
Patient satisfaction score was significantly higher in groups M and H compared with group L; however, there was no difference between groups M and H in this instance (Table 1). None of the patients needed analgesic drugs at 2 and 24 hours postoperatively. On average, patients in group L needed more acetaminophen injection at 4 and 8 hours postoperatively in comparison with groups M and H. The difference in the average number of acetaminophen injections was not significant among the three groups at 12 hours postoperatively. The postoperative acetaminophen consumption in group L was higher compared with groups M and H at 4 and 8 hours postoperatively (P < 0.02). The mean dose of injected acetaminophen in the first 24 hours was 1370 mg, 846 mg, and 615 mg in groups L, M, and H, respectively. The consumed acetaminophen was significantly higher in group L in comparison with groups M and H, whereas the difference between groups M and H was not statistically significant (Table 1). The average number of morphine injections (as a rescue dose) did not have a significant difference among the three groups at any time point (Table 1).
| Variables | Group L (n = 27) | Group M (n = 26) | Group H (n = 27) | P-Value |
|---|---|---|---|---|
| Age (y) | 48.8 ± 14.9 | 45.5 ± 13.8 | 52.7 ± 12.6 | 0.17 |
| BMI | 26.7 ± 2.1 | 25.3 ± 3.1 | 26.8 ± 2.9 | 0.16 |
| Duration of surgery (h) | 0.80 ± 0.25 | 0.85 ± 0.21 | 0.84 ± 0.28 | 0.76 |
| Duration of anesthesia (h) | 1.9 ± 0.42 | 1.9 ± 0.31 | 2.0 ± 0.4 | 0.68 |
| VAS score when coughing | ||||
| 2 h postoperatively | 2.2 ± 1.1 | 0.4 ± 1 | 0.1 ± 0.6 | < 0.001 |
| 4 h postoperatively | 4.7 ± 1.3 | 2.7 ± 0.9 | 2.8 ± 0.7 | < 0.001 |
| 8 h postoperatively | 5.2 ± 1.3 | 4.8 ± 1.2 | 4.3 ± 1 | < 0.001 |
| 12 h postoperatively | 4.5 ± 1.2 | 4.8 ± 1.2 | 4.6 ± 1.5 | 0.70 |
| 24 h postoperatively | 2.3 ± 0.6 | 2.1 ± 0.5 | 1.8 ± 0.5 | 0.10 |
| Patient satisfaction scores | 0.04 | |||
| Very dissatisfied | 0 | 0 | 0 | |
| Dissatisfied | 4 (14.8) | 0 | 1 (3.8) | |
| Neither satisfied nor unsatisfied | 8 (29.6) | 5 (19.2) | 3 (11.5) | |
| Rather satisfied | 15 (55.6) | 13 (50) | 16 (63.5) | |
| Completely satisfied | 0 | 8 (30.8) | 6 (21) | |
| Postoperative acetaminophen consumption | ||||
| 2 h postoperatively | 0 | 0 | 0 | |
| 4 h postoperatively | 11 (40.7) | 1 (3.8) | 0 | < 0.001 |
| 8 h postoperatively | 16 (59.3) | 10 (38.5) | 6 (23.1) | 0.02 |
| 12 h postoperatively | 10 (37) | 11 (42) | 10 (38) | 0.92 |
| 24 h postoperatively | 0 | 0 | 0 | |
| Postoperative morphine consumption (h) | ||||
| 2 | 0 | 0 | 0 | |
| 4 | 2 (7.4) | 0 | 0 | 0.32 |
| 8 | 5 (18.5) | 2 (7.7) | 1 (3.8) | 0.25 |
| 12 | 1 (3.7) | 2 (7.7) | 2 (7.7) | 0.73 |
| Adverse effects | ||||
| Bradycardia | 0 | 0 | 1(3.8) | < 0.001 |
| Sedation (based on Ramsay score 2 or 3) | ||||
| Postop 0 h | 3 (11) | 26 (100) | 26 (96) | < 0.01 |
| Postop 2 h | 0 | 24 (92) | 24 (88) | < 0.01 |
| Postop 4 h | 0 | 8 (29) | 14 (51) | < 0.01 |
Demographic and Clinical Features of the Groups a
The patients in groups M and H had a higher modified Ramsay score and sedation duration in comparison with group L at 0, 2, and 4 hours postoperatively (Table 1). None of the patients in any of the groups had nausea or vomiting. In the assessment of heart rate within groups, only one patient (who belonged to the H group) had bradycardia. The bradycardia happened immediately after the block and was treated with 0.7 mg atropine. The mean blood pressure was significantly lower in group H in comparison with groups L and M at 4 and 12 hours postoperatively (P < 0.001) (Table 1).
Patients with surgical mesh received significantly more analgesics than the non-mesh group, which was dose-dependent [H (89%) < M (81%) < L (52%)] (P = 0.03).
5. Discussion
We found that supplementation of bupivacaine with 1 µg/kg of dexmedetomidine in TAP block provided longer postoperative analgesia, lower VAS score, and lower analgesic consumption than the 0.5 µ/kg over 8 hours compared with 1.5 µg/kg with fewer side effects, such as drowsiness and bradycardia.
In a study by Neethirajan et al. (13) 60 patients who were scheduled for laparoscopic appendectomy under general anesthesia were randomly divided into two groups. Before the beginning of the surgical incision, they underwent a TAP block using 20 mL of 0.125% bupivacaine that was supplemented with 1 μg /kg dexmedetomidine or 2 mL normal saline. They concluded that the addition of dexmedetomidine to bupivacaine in TAP block produces more postoperative pain-free time, provides better analgesia, and reduces rescue analgesic requirements in comparison with bupivacaine alone.
In their study, Chen et al. (14) compared the effect of adding dexmedetomidine or fentanyl into ropivacaine in TAP block on analgesic efficacy and recovery quality in 100 elective gynecological patients. They performed TAP blocks postoperatively, using 0.375% ropivacaine plus dexmedetomidine 1 µg/kg in one of four groups. They concluded that consumption of dexmedetomidine as a supplement to TAP blocks might facilitate postoperative analgesia and advance the value of recovery. In terms of dexmedetomidine dose, the results of the TAP block in these two studies are consistent with our Study.
In another study, Almarakbi et al. (15) randomly assigned 50 patients who were scheduled for abdominal hysterectomy into two groups. They did bilaterally TAP block using 20 mL of 0.25% bupivacaine that was supplemented with 0.5 mcg/kg (2 mL) of dexmedetomidine or 2 mL of normal saline in the experimental or control groups, respectively. They concluded that VAS was significantly lower in the experimental group in comparison with the control group in the first eight postoperative hours, both while resting and coughing.
In a study by Shehab et al. (16) in 3 groups of 25 patients who were candidates for abdominal and pelvic cancer surgery, after closing the skin and completing the surgery under general anesthesia, TAP block was performed, and paracetamol was added to the postoperative drugs. In the first group (TAP) 30 mL bupivacaine 0.25%, in the second group (TAP + DEX), 30 mL bupivacaine 0.25% + 0.5 mcg/kg dexmedetomidine, and in the third group, (TAP + IV-DEX) 30 mL bupivacaine 0.25% in block + IV dexmedetomidine were used. The first analgesic request time for TAP was 1.6 ± 5.7 hours, and for TAP + DEX, 2.9 ± 9.8 hours. Morphine consumption in the first 24 hours for TAP was 24mg and for TAP + DEX was 11 mg. The VAS score in the TAP group increased 6 hours later, and in the two DEX groups, 12 hours later. This study revealed that the use of whether topically or intravenously dexmedetomidine provided a deeper postoperatively analgesia and lesser extra analgesic consumption. In a study by Ramya et al. (17) in two groups of 35 patients, TAP block was performed after cesarean section by spinal anesthesia. In group B, 20 ml of 0.25% bupivacaine was used, and in group BD, 20 mL of bupivacaine 0.25% and 0.5 µ/kg dexmedetomidine was used, and patients were given paracetamol (1 g) immediately after block and every 8 hours in the ward. The duration of the block in group BD was 14 hours compared with 8 hours in group B. The VAS score and morphine consumption in the first 24 hours in the BD group were lower than in the B group. They concluded that the addition of dexmedetomidine to bupivacaine in TAP block could prolong the time to request the first dose of rescue analgesia and also reduced the total dose of opioid requirement in the first 24-h after cesarean section.
All three above-mentioned studies used 0.5 mcg/kg of dexmedetomidine as a supplement drug to bupivacaine in TAP block. Unlike these studies, the maximum duration of block effect with 0.5 mcg/kg of dexmedetomidine was 4 hours in the present study, which may be due to the different designs of the above studies. These differences include performing TAP block before surgical incision in Almarakbi study, the use of a high volume of the drug (30 mL) in Nahla study, and the simultaneous use of analgesics in both Nahla and Ramya studies. Bupivacaine concentration was twice as our study, as well. Aksu et al. (18) divided 93 patients who were submitted for lower abdominal surgery into three groups, and TAP block was performed after general anesthesia induction but before surgical incision. In the control group (C), for block, 20% mL saline 0.9% and in group B, 20 mL bupivacaine 0.5%, and in group BD, 20 mL bupivacaine 0.5% with 1.5 mcg/kg dexmedetomidine were used. The results of this study showed that the VAS score was lower in the BD group than in the control group during the 8 hours after the operation. While the duration of the block in group B was 8 hours, adding dexmedetomidine increased the duration of the block to 24 hours. Morphine intake was lower in the other two groups up to 24 hours after block, and sensory block in the BD group was lower than in the other two groups. In the present study, in contrast to the mentioned study, the duration of effect of 1.5 mcg/kg dexmedetomidine was a maximum of 8 hours, which could be due to the difference in the concentration of bupivacaine (0.5 vs. 0.125%). However, the results of the VAS score in the first 8 hours and blood pressure and morphine consumption 24 hours after surgery in the current study were consistent with the study by Aksu et al..
In most studies performed so far, patients have been subjected to general anesthesia, and the TAP block performed after general anesthesia and before surgery (18). The drowsiness of patients after waking up have also been attributed to general anesthesia. Ramya et al. (17) used spinal anesthesia and did not indicate that patients fell asleep. In the present study, due to the fact that TAP block was performed by spinal anesthesia at the end of surgery and no sedative drug was prescribed during the operation, patients clearly experienced sedation and drowsiness, which were seen in group L for up to 2 hours and in groups M and H up to 4 hours, and in some cases, drowsiness, especially in group H, caused latency in patients’ discharge from the recovery ward.
5.1. Limitation
Given that the sedation score in the M and H groups was higher than the L group, a medium dose of dexmedetomidine may be associated with better analgesic effect. However, we did not test the medium dose, which is one of the limitations of our study.
5.2. Conclusions
The use of 1 and 1.5 µ/kg dexmedetomidine in the TAP block provides a longer analgesic effect and reduces the need for postoperative analgesics in comparison with its use at 0.5 µ/kg. However, dexmedetomidine at 1.5 µ/kg causes more adverse effects, like drowsiness, bradycardia, and lower MAP than dexmedetomidine at 1 µ/kg. According to the results of this study, the appropriate dose of dexmedetomidine for adding bupivacaine in the TAP block is 1µ/kg.