1. Background
Tracheal extubation is critical and causes stressful moments for patients during emergence from general anesthesia. It may induce undesirable and even hazardous hemodynamics and airway responses, such as tachycardia, hypertension, dysrhythmias, coughing, laryngospasm, and bronchospasm (1, 2). These hazardous responses are caused by the sudden release of catecholamines during tracheal extubation (3). Many medications have been studied to reduce the stress response during extubation, aiming to achieve a state of smooth extubation, such as intravenous lignocaine, short-acting opioids as fentanyl, remifentanil, esmolol, endotracheal local anesthetic instillation, and dexmedetomidine (4-9). Also, a novel method was used to achieve fast-track anesthesia with integrated inhalational-intravenous anesthesia with sufentanil infusion and using various anesthesia monitoring systems that permit lowering the dose of the whole anesthetics and preserving the reasonable anesthetic depth and hemodynamic stability during simple cardiac surgery with cardiopulmonary bypass. Thus, extubation of the trachea in the operating room is possible in these patients without complications (10). Propofol (2, 6-diisopropyl phenol) is a sedative and hypnotic drug with antiemetic properties used for induction and maintenance of general anesthesia and sedation in intensive care units (ICU). It produces its effect by facilitating inhibitory neurotransmission mediated by gama amino butyric acid (GABA) depression (11-13). Ketamine is a phencyclidine derivative used for inducing general anesthesia. It has favorable analgesic and amnestic properties but increases sympathetic activity, nausea, vomiting and may cause undesirable psychomimetic disorders (14, 15). Ketofol (ketamine-propofol mixture) is an induction agent, and its effect on hemodynamics has been studied previously (16, 17). Aberra et al. studied Ketofol’s effect on airway responses during inducing anesthesia (18). We hypothesized that giving ketofol as an induction agent with the beneficial effects of both medications (Ketamine and propofol ) would favorably affect both hemodynamics and the airway response during extubation.
2. Objectives
This study aimed to examine the effect of injecting a single dose of ketofol and propofol on the smoothness of extubation regarding the airway response and hemodynamics when given for inducing general anesthesia in laparoscopic drilling of polycystic ovary disease.
3. Methods
The Ethical Committee of the Anesthesia Department, the Fayoum University (R83), approved the study and written informed consent was obtained from the patients. This study was conducted on 106 female patients with the American Society of Anesthesiologists Physical Status ''ASAPS'' class I and II, aged 18 - 40 years, who were scheduled for laparoscopic drilling for polycystic ovary under general anesthesia. This trial protocol was registered at clinical trial.gov with the identification number NCT04365686. This study was conducted in the Fayoum University’s hospitals in 12 months between April 2019 and April 2020. Patients suffering from cardiac diseases, hepatic diseases, renal diseases, and history of epilepsy were excluded from the study. The patients were randomly allocated into one of the two study groups using a computer-generated table. The randomization sequence was concealed in opaque sealed envelopes. The authors opened the envelopes after recruitments and admission to the operation room. Only assessors and data collectors were blinded to the group's allocations. Our study adhered to the CONSORT guidelines. Study groups in this randomized, double-blind, parallel clinical trial were as follows: Group KP (Ketamine-Propfol Mixture Group) including 53 female patients receiving propofol (1 mg/kg) plus ketamine (0.5 mg/kg) at the induction of general anesthesia and Group P (propofol group) including 53 female patients receiving propofol (2 mg/kg) at the induction of general anesthesia.
After securing intravenous access by a 20 g I.V. cannula, intravenous premedication (midazolam 2 mg and 4 mg ondansetron) was administered to all the patients. Standard ASA monitoring (5-lead ECG, non-invasive blood pressure (NIBP), and pulse oximetry) was applied to all the patients for recording the heart rate (HR), NIBP, and oxygen saturation using a multi-parameter monitor. General anesthesia was induced as follows: in Group KP (Ketamine-Propfol Mixture Group), 53 female patients received propofol (1 mg/kg) plus ketamine (0.5 mg/kg) at the induction of general anesthesia, while in Group P (Propofol Group), 53 female patients received propofol (2 mg/kg) only at the induction of general anesthesia. Patients in both groups received intravenous fentanyl 2 µg/kg and atracurium 0.5 mg\kg. After tracheal intubation, general anesthesia was maintained by isoflurane 1 - 1.5 % in 2 L/min oxygen-air mixture 50% : 50% or as required to keep stable hemodynamics and atracurium 0.1 mg/kg every 30 min, if needed. At the end of the surgery, inhalational anesthesia was stopped, and within 1 min after stopping inhalational anesthesia, the neuromuscular blockade was reversed by intravenous neostigmine 0.05 mg/ kg and atropine 0.01 mg/kg. Hemodynamics [HR and mean arterial blood pressure (MAP)] was assessed at 5-min intervals from the time of muscle relaxant reversal (about 5 minutes before the expected extubation time) up to 20 min after extubation. The sedation level during oral suction and extubation was assessed using an observer assessment sedation score (Table 1), and the airway response to oral suction was noted using a five-point scale (Table 2) (19). Time at which oral suction for all operations was done ranged from 2 to 3 minutes and was not significant. The level of sedation and smoothness of extubation was noted using a four-point scale (Table 3) (19). Other secondary outcomes as spontaneous speaking, hallucination, confusion, and delerium were noted from extubation time to 2 hours postoperative.
| Observation | Score |
|---|---|
| Responds readily to name spoken in a normal tone | 5 |
| Lethargic response to a name spoken in a normal tone | 4 |
| Responds only after a name is called loudly and\or repeatedly | 3 |
| Responds only after mild podding or shaking | 2 |
| Does not respond to mild podding or shaking | 1 |
| Grade | Description |
|---|---|
| 1 | Excellent (breathing well, no response to suctioning) |
| 2 | Good (breathing well, minimal grimacing response to suctioning) |
| 3 | Satisfactory (breathing well & coughing attempt to suctioning) |
| 4 | Poor (breathing well, mild coughing on the tube) |
| 5 | Very poor (breathing well, aggressive coughing on the tube) |
| Grade | Description |
|---|---|
| 1 | No coughing on the endotracheal tube |
| 2 | Coughing on the tube |
| 3 | Vomiting |
| 4 | Laryngospasm |
The primary objective was to assess the smoothness of extubation, while the secondary objective was to assess the airway response to suction, sedation score, hemodynamics, spontaneous speaking, hallucination, delerium and confusion, as mentioned before.
3.1. Sample Size Calculation
Our pilot study was carried out on 20 patients randomly allocated using blind envelope methods into two groups, each with ten patients. These patients were not included in the final study. The sample size was calculated using the G* power version 3.0.10. The minimal sample size was calculated to be 48 patients in both groups, needed to obtain the power level of 0.80, the alpha level of 0.05 (two-tailed), and the effect size of 0.58 for grading the smoothness of extubation. Grade 1 (mean ± SD) was1.5 ± 0.8 and 2.0 ± 1.2 in the study group and the control group, respectively. Based on the pilot study's results on overcoming the follow-up loss, the calculated sample size increased by 10% to reach 53 in each group.
3.2. Statistical Analysis
Statistical analysis was performed using SPSS version 23. Descriptive statistics were carried out for categorical variables and presented as numbers and percentages. For numerical data, descriptive statistics were performedusing mean & standard deviation. The independent t-test was used to compare numeric variables in the two groups being either normally distributed or based on the central limit theorem. The Chi-Square test was used to analyze the difference in the extubation quality, the sedation score, and the adverse events. P-value< 0.05 was considered statistically significant.
4. Results
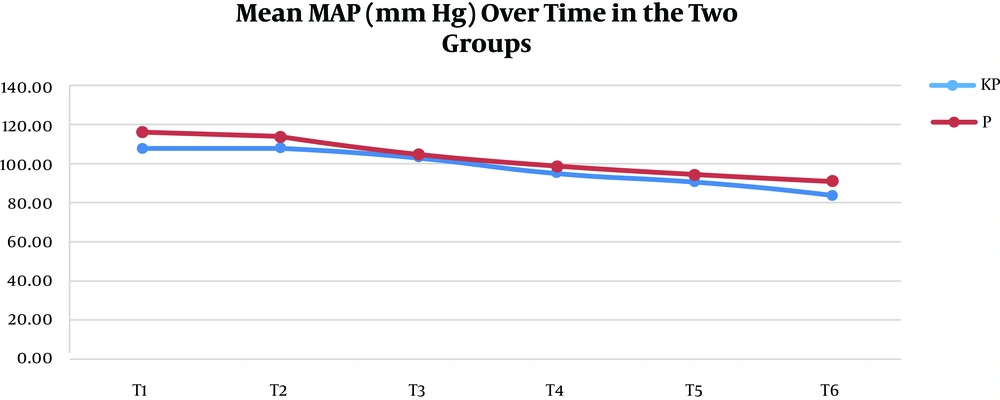
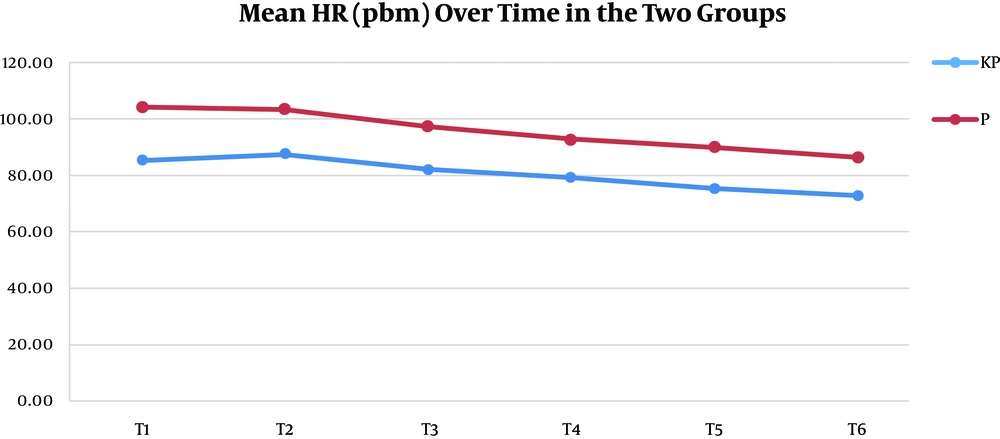
A total of 106 patients participated in this study. They were randomized into the K group (53 patients) and the P group (53 patients). All the patients completed the study, and there were no dropouts (Figure 1). There were no statistically significant differences between the two study groups regarding the demographic data, duration of surgery, duration of anesthesia, and postoperative meperidine consumption except for the clinically insignificant height (Table 4). Hemodynamic parameters (MAP and HR), 5 min before expected extubation (T1), at the time of extubation (T2), and every 5 min later up to 20 min post-extubation (T3-T6), are detailed in Figures 2 and 3.
| Parameters | Ketofol (KP) Group (N = 53) | Propofol (P) Group (N = 53) | P-Valueb |
|---|---|---|---|
| Age (y) | 27.68 ± 3.67 | 26.49 ± 3.06 | 0.073c |
| Height (cm) | 164.13 ± 5.20 | 166.25 ± 5.05 | 0.036 c |
| Weight (kg) | 74.42 ± 10.19 | 73.36 ± 7.92 | 0.552c |
| BMI kg/m2 | 27.70 ± 4.18 | 26.56 ± 2.80 | 0.103c |
| Surgical time (min) | 23.5 ± 4.3 | 25.1 ± 5.1 | 0.079c |
| Anesthesia time (min) | 31.4 ± 5.4 | 33.2 ± 6.2 | 0.118c |
| Meperdine consumption | 0.148d | ||
| 0 | 32 (60) | 31 (59) | |
| 25 | 18 (34) | 13 (25) | |
| 50 | 3 (6) | 9 (17) |
a Values are expressed as mean ± (SD) or No. (%)
b P-value is significant at or below 0.05.
c Using the independent t-test.
d Using the chi-square test.
MAP was significantly higher in the P group than in the KP group at all readings except at 5 min after extubation, where the difference was non-significant (Figure 2). HR was significantly lower in the KP group than in the P group at all readings (Figure 3). The grade of the smoothness of extubation, our primary outcome, was 1 in all the KP group cases but 2 to 4 in the P group cases. This relationship was highly significant, and the Somers' D value = 1, meaning that there was a perfect trend association (Table 5). The sedation level during the suction process was 1 in 83% of the KP group cases, with lower numbers at levels 2 and 3. However, the level was 1 in 19% of the P group cases and 2 and 3 in the rest of the cases. This relationship was highly significant, and the Somers' D value = 0.67, meaning that there was a strong trend association (Table 5).
| Group | Ketofol (K Group)a | Propofol (P Group)a | The P-Valueb | Somers' D Value (A Measure of Trend) |
|---|---|---|---|---|
| Level of sedation during suction | < 0.001c | 0.675 | ||
| 1 | 44 (83) | 10 (18.9) | ||
| 2 | 7 (13.2) | 23 (43.4) | ||
| 3 | 2 (3.8) | 20 (37.7) | ||
| Airway reflex | < 0.001c | 0.553 | ||
| 2 | 11 (20.8) | 2 (3.8) | ||
| 3 | 33 (62.3) | 12 (22.6) | ||
| 4 | 5 (9.4) | 33 (62.3) | ||
| 5 | 4 (7.5) | 6 (11.3) | ||
| Level of sedation at extubation | < 0.001c | 1 | ||
| 2 | 7 (13.2) | 0 | ||
| 3 | 46 (86.8) | 0 | ||
| 5 | 0 | 53 (100) | ||
| Smoothness of extubation | < 0.001c | 1 | ||
| 1 | 53 (100) | 0 | ||
| 2 | 0 | 20 (37.7) | ||
| 3 | 0 | 30 (56.6) | ||
| 4 | 0 | 3 (5.7) |
aValues are expressed as No. (%).
bUsing the chi-square test.
c P-value is significant at or below 0.05.
The grade of airway reflex was 2 in 11 cases (20.8%) and 3 in 33 cases (62.3%) of the KP group with lower numbers at grades 4 and 5. While in the P group, 12 cases (22.6%) were in grade 2 and 33 cases (62.3%) were in grade 4. Also, this relationship was highly significant, and the Somers' D value = 0.55, meaning that there was a strong trend association (Table 5). The sedation level at extubation was 5 in all the P group cases but 2 and 3 in the KP group cases. This relationship was highly significant, and the Somers' D value = 1, meaning that there was a perfect trend association (Table 5).
5. Discussion
Stress response during extubation is an unwanted and unpredictable response that makes anesthesiologists vigilant and attentive for minimizing its effect on hemodynamics and airway reflexes. In our study, we examined the effect of injecting a single dose of ketofol compared to propofol at the induction of general anesthesia on the smoothness of extubation regarding airway response and hemodynamics in laparoscopic drilling of polycystic ovary disease. Aboeldahab et al. studied the effect of ketofol compared to its two constituents on 60 patients undergoing hernia repair under general anesthesia. They clinically examined ketofol‘s effect as an induction agent by assessing hemodynamics and using the bispectral index (BIS). They gave ketofol to 20 of the patients , propofol to 20 of them, and ketamine to the last 20 during inducing anesthesia. During extubation, HR was lower in the ketofol group (81.65 ± 2.60) than in the propofol group (81.73 ± 3.93) with no statistical significance, and MAP was lower in the ketofol group (83.90 ± 3.30) than in the propofol group (85.66 ± 3.43) with no statistical significance. These results might be due to the lengthy procedure and/or the small sample size in their study. Our results are in agreement with those of Aboeldahab et al., as ketofol was associated with more stable hemodynamics than propofol during extubation. Also, Sabertanha A et al., in their study, deduced that the infusion of a combination of ketamine and propofol increased hemodynamic stability and was superior in analgesia compared to the infusion of only propofol (20). We attributed this to the good sedation level of ketofol during suction and extubation due to the additive analgesic and sedative effect of ketamine and propofol. We found that ketofol’s effect on HR was more significant in stabilizing hemodynamics than its effect on MAP when compared to propofol. Jalili et al. compared the effect of propofol and ketofol on the emergence of delirium in 87 ASA I and II children aged 3 - 12 years undergoing adenotonsillectomy. They reported a statistically non-significant difference between the two groups regarding HR in the recovery room at 0, 10, and 20 min postoperatively (21). The smoothness of extubation without coughing, laryngospasm, and vomiting on the tube were examined in both groups, and it was in favor of the KP group. Aberra et al. conducted a study on 120 pediatric patients aged 2 - 15 years undergoing elective ophthalmic surgical procedures under general anesthesia using laryngeal mask airway (LMA) to compare the ketamine–propofol mixture (ketofol) with propofol only on the ease of laryngeal mask airway insertion conditions and hemodynamic effects during inducing general anesthesia. They found that 54 patients in the ketofol group compared to 52 in the propofol group developed no cough. They also realized that six patients in the ketofol group compared to seven patients in the propofol group developed a slight cough (coughing which can occur immediately after LMA and subside by itself), and one patient in the propofol group developed a gross cough (coughing that needs deepening of anesthesia to be relieved) with no significant difference between them (18). We also reported that the sedation scores during suction and extubation were significantly lower in the KP group compared to the P group. We attributed this good sedation level during suction and extubation to the analgesic effect of ketamine. In our study, we observed that the KP group showed a better airway response than the P group. One of the most adverse effects of the stress response on the airway is cough. Kim and Bishop reported a 75% incidence rate of cough in patients during emergence and extubation (22). Hypertension, tachycardia, myocardial ischemia, and bronchospasm are adverse effects related to cough. In our study, the majority of patients in the KP group [44 patients (83.1%)] developed better airway reflexes (grade 2 or 3) during suction than the majority of patients in the P group [39 patients (73.6%)], as they developed a higher degree of airway reflexes (grade 4 or 5) that subsides by itself in grade 4 and needs deepening of anesthesia in grade 5. We gave a different explanation for the better hemodynamic stability during the extubation process in the KP group from all the previous ones. Aberra et al. reported that no patient in the ketofol group developed laryngospasm, while two patients in the propofol group developed partial laryngospasm with no statistical significance. They concluded that Ketofol provided equivalent laryngeal mask airway insertion conditions, and that it could be used as alternative propofol for LMA insertion (18). Patients in the KP group showed a higher sedation level during the suctioning and extubation procedures when compared to those of the P group. The reason may be more sedative and more decreasing of the airway reflexes of ketofol during recovery in such short surgical procedures.ketamine was analgesic at low concentration and anesthetic at high concentration. At low concentrations, analgesic properties are obvious, whereas at higher concentrations, anesthetic properties become superior (23). Rani et al. showed a degree of sedation with dexmedetomidine 0.75 μg/kg during recovery compared to fentanyl 1 μg/kg when given 15 min before the end of the surgery (19). Also, El Mourad MB et al. reported that ketofol was more superior in offering rapid onset of sedation, lower intubation time, more stable hemodynamics, and more satisfaction for anesthesiologists when compared to dexmedetomidine-propofol (24). Ketofol, with decreased doses of both drugs, possesses the analgesic effects of ketamine and decreasing airway reflexes of propofol with hemodynamic stability, i.e. it keeps the benefits of each drug and excludes unwanted effects (the analgesia of ketamine without increasing airway reflexes or sympathetic stimulation and the depressive effect of propofol on airway reflexes without hemodynamic instability) due to the additive effect of GABA agonism by propofol and N-Methyl D-Aspartate (NMDA) antagonism by ketamine. This may explain more hemodynamic stability, smoothness of extubation, decreased airway reflexes, and more sedative effect during suctioning and recovery in the KP group without spontaneous speaking, delerium, confusion, and hallucination in both groups.
5.1. Conclusions
Ketofol during inducing general anesthesia in laparoscopic drilling of polycystic ovary provided a good sedation score during suction and extubation. a better airway response during suction, smoothness of tracheal extubation, and more stable hemodynamics were obtained than when propofol was only given.
5.2. Limitation and Recommendations
We performed this study in a relatively short surgical procedure that could be a limitation of our study. We did not measure the recovery time directly to detect if there was any significant difference. Also, we followed up with the patients for a relatively short time (20 min only). We recommend comparing the effect of both drugs on extubation and recovery from anesthesia in longer duration surgeries or giving ketofol 15 min before the end of the surgery at various durations.