1. Background
Traumatic brain injury (TBI) is a common cause of disability worldwide. In fact, trauma is the second most common cause of death and disability (1, 2). Each year, more than 10 million people develop TBI worldwide. In the United States, the estimated incidence of TBI is between 180 and 250 cases per 100,000 people. In Europe, about 235 cases per 100,000 people are hospitalized each year due to TBI (3, 4). In the old member states of the European Union and in the United States, approximately 7.7 million and 5.3 million people live with some form of TBI disability, respectively (3). People who survive after TBI, in addition to cognitive impairment, suffer from motor disorders and spasticity (5), which negatively affects their performance and quality of life (6). Spasticity accompanying orthopedic complications usually occur one week after onset of TBI (7).
Spasticity can be broadly defined as ‘sensory-motor disorder caused by upper motor neuron lesions that manifests as involuntary intermittent or continuous muscle activation’ (8). Spasticity symptoms include increased muscle tone, muscle contraction, increased deep tendon reflexes, clonus, and joint immobilization (9). Spasticity can range from mild muscle stiffness to uncontrollable severe muscle contraction. In addition to the symptoms, spastic musculoskeletal problems such as muscle weakness decreased control of movement, and decreased stability may occur (10). According to the existing evidence, the incidence rate of muscle deformity due to spasticity is 85% (11). Shortly after a brain injury, most patients experience a period of increased muscle tone. A common condition is that elbows are placed in the lateral, and the wrists and fingers are bent (clenched fists). Spasticity may be very mild, with a feeling of tightness in the muscles, or very severe, with painful and uncontrollable spasms of the limbs, often in the legs and arms. The adverse effects of spasticity include muscle stiffness that impairs function, uncontrollable muscle contractions, which are often painful, muscle and joint deformity, inhibition of muscle longitudinal growth, and inhibition of protein synthesis in muscle cells. Other organ-related complications include urinary tract infections, chronic constipation, and pressure sores (12).
Non-penetrating head injury causes more severe spasticity than spinal cord injury, especially in thoracic segments (1, 13). Although spasticity measurement is a problem, the improvement of treatment methods will face obstacles without its measurement (14). Spasticity should be evaluated objectively to track its development over time. For this purpose, various scales have been designed and validated (15). The Modified Ashworth Scale (MAS) and the Modified Tardieu Scale (MTS) are among the most common scales used. MAS measures the level of resistance to a passive movement. This scale is widely used in both research methods and clinics due to its quick and easy use (16). Patient spasm assessment by using valid and sensitive tools is a very important step to develop and adjust the most effective antispasmodic treatment (17, 18).
There are several treatments for spasm management and treatment (17). Therapeutic goals in spasticity include relieving spasticity symptoms, reducing pain and muscle contraction frequency, as well as improving gait, health, and daily activities (19). One of the most common treatments for spasticity is the use of medications. Most pharmacological treatments aim to reduce the release of stimulant neurotransmitters (e.g., glutamate and monoamines) or increase the release of inhibitory neurotransmitters [glycine and γ-aminobutyric acid (GABA)] and reduce reflex activity (20, 21).
Demyelination after peripheral nerve damage is associated with abnormalities of sodium channels and spontaneous activity of the fibers, and these changes can occur in the central nervous system after TBI. Thus, sodium channel blocks are thought to be another way to reduce spasticity. The reversible block of sodium channels is a pharmacological feature of topical anesthetic drugs, and drugs such as bupivacaine and lidocaine have been used intrathecally, intravenously, and subcutaneously to treat spasticity (22, 23). Lidocaine is one of the drugs used to prevent the onset of action potential and conduction in excited neural tissues (24, 25).
The drug has a central sedative-analgesic effect and blocks ion transport by blocking sodium channels, thus preventing the onset of action potential and conduction in excitable tissues (20). If the drug is administered through the nares to the olfactory mucosa, the drug molecule can pass directly through this tissue and enter the cerebrospinal fluid. Olfactory mucosa is located in the upper part of the nasal cavity, just below the cribriform plate, which contains olfactory cells. When drug molecules come in contact with this mucosa, they are quickly and directly transmitted to the brain by bypassing the blood-brain barrier, and their levels in the cerebrospinal fluid (CSF) rise rapidly (often faster than intravenous administration). This concept of transfer of molecules from the nose to the brain is called the nose-to-brain pathway and is used for centrally acting drugs, including sedatives, anticonvulsants, and narcotics. Since 1996, intranasal administration of lidocaine in various concentrations has been used successfully in the treatment of migraine and trigeminal neuralgia (26-28).
Given the problems with drugs used in the treatment of spasticity, including the effectiveness and side effects of these drugs, it is important to find a right drug for rational prescribing.
2. Objectives
This study aimed to investigate the effect of intranasal lidocaine 0.5% for the treatment of spasticity in patients with TBI.
3. Methods
Due to the novelty and pilot of this study, it was decided to conduct the study as a single-arm research and evaluate the effects of the drug on each patient separately. This single-arm study was performed with a pretest-posttest design. Due to the lack of similar studies and according to the average number of patients with spasticity following TBI during two years before the outset of the study, the study was performed as a pilot study on 15 patients with spasticity following TBI during one year. After receiving ethical approval from the Ethics Committee of Guilan University of Medical Sciences (IR.GUMS.REC.1397.212) and the thesis registration code number (1011), a full explanation of the study was provided to the legal guardians of the patients participating in the study. Sampling was performed based on the census method, and participants were enrolled after obtaining written informed consent.
Patients with moderate and severe spasticity following TBI in the age group of 16 to 55 years admitted to the intensive care unit (ICU) of Poursina Hospital in Rasht, Iran entered the study. The latest edition of Guidelines for the Management of Severe Traumatic Brain Injury for patient with TBI (29) was implemented equally for all the patients. Before starting the study, the medical history of all patients was assessed and examination of the head and neck for polyps and other intranasal lesions was performed.
Initially, for each patient with spasticity, 25 mg of oral baclofen 3 times a day (maximum daily dose) was started as a part of standard therapy. After 48 hours, lidocaine treatment was started if spasticity did not reduce by at least one score according to the MTS or MAS.
The continuous infusion of lidocaine 0.5% began with the initial dose of 1 mg/min and gradually increased to 2 mg/min according to the patient’s response. For intranasal infusion of lidocaine, the usual infusion pump in the ICU and the nasal cannula as an interface were used. There was no evidence of drug returning out of the nose during the study. The patients were evaluated for visible signs of drug toxicity, and in case of complications such as bradycardia, dysrhythmia, drowsiness, and confusion, the infusion was discontinued, and monitoring performed until symptoms resolution. For all the patients, monitoring of blood pressure (BP), heart rate (HR), and arterial oxygen saturation (SPo2) was performed continuously. The sedation score maintained the same in all the patients and was checked and recorded four times a day by the Richmond agitation-sedation scale (RASS).
The target of the sedation score was between -4 and +1. Patients were assessed daily for changes in the level of consciousness by the Glasgow Coma Scale (GCS). Spasticity was assessed by MTS and MAS. Also, spasm frequency was assessed by Spasm Frequency Score (SFS), and patients with a score above 3 were evaluated. The positive response to treatment in this study was defined as a reduction of at least 2 spasticity scores. The study duration for each patient was 9 days. The exclusion criteria included use of muscle relaxant drugs; the presence of a neurological disease that interferes with spasticity assessment; drug users; pregnant or lactating women; significant laboratory abnormality including severe electrolyte disturbances refractory to therapy (potassium less than 3.5 and above 5 meq/L, magnesium less than 1.5 and above 2.6 mg/dL, and calcium less than 8 and above 11 mg/dL); severe acidosis and alkalosis; severe hemodynamic disturbances requiring sedation or analgesia more than the amount specified in the study, which reduces the level of consciousness; and allergy to topical anesthetic agents, including lidocaine. Lidocaine precautions include heart disease, liver failure, base of skull fractures, and a positive response to baclofen therapy. Due to the unavailability of a blood or CSF measurement kit for lidocaine in Iran and the impossibility of obtaining it from abroad, it was decided to use the minimum daily intravenous dose for intranasal treatment. Time limit for lidocaine use in case of non-toxic dosing is not mentioned in the pharmacological and cardiac source (30, 31).
3.1. Statistical Analysis
After collecting the data, all data was analyzed by SPSS software version 21. Mean, standard deviation, and percentage of the frequency were used to describe the data. A paired t-test was used to compare the differences in quantitative variables at the beginning and end of treatment. P-value less than 0.05 was considered as statistically significant.
4. Results
Out of 15 participants in this study, 13 (86.7%) were male, and 2 (13.3%) were female. The age range of participants was 29.26 ± 12.5 years (minimum: 15 years and maximum: 54 years). Paired t-test showed no statistically significant differences in MAS, MTS, RASS, GCS, mean arterial blood pressure (MAP), SPo2, HR, respiratory rate (RR), and number of spasms per day between the time of treatment initiation and the second day of baclofen treatment (P > 0.05; Table 1).
| Variables/Time | Mean ± SD | Test Value | P-Value |
|---|---|---|---|
| Modified Ashworth Scale | - | - | |
| Onset of treatment | 3.46 ± 0.51 | ||
| Second day | 3.46 ± 0.51 | ||
| Modified Tardieu Scale | 1.46 | 0.0164 | |
| Onset of treatment | 3.46 ± 0.51 | ||
| Second day | 3. 6±0.5 | ||
| RASS | 1.97 | 0.068 | |
| Onset of treatment | -1.86 ± 0.91 | ||
| Second day | 1.4 ± -1.45 | ||
| GCS | 1.74 | 0.104 | |
| Onset of treatment | 10.26 ± 2.12 | ||
| Second day | 10 ± 2.23 | ||
| MAP | 0.346 | 0.735 | |
| Onset of treatment | 96.73 ± 11.49 | ||
| Second day | 97.33 ± 12.87 | ||
| SPO2 | 1.79 | 0.095 | |
| Onset of treatment | 96.93 ± 1.75 | ||
| Second day | 97.53 ± 1.18 | ||
| Heart rate | 0.673 | 0.512 | |
| Onset of treatment | 91.8 ± 14.7 | ||
| Second day | 93.06 ± 10.81 | ||
| Respiratory rate | 0.153 | 0.88 | |
| Onset of treatment | 24.06 ± 3. 8 | ||
| Second day | 23.93 ± 3.93 | ||
| The average number of spasms per day | 0 | 1 | |
| Onset of treatment | 13.13 ± 3.7 | ||
| Second day | 13.13 ± 3.88 |
Paired t-test revealed a significant decrease in the mean scores of MAS and MTS and the frequency of spasms at the end of treatment (day 9) compared to the time of treatment initiation (P < 0.0001; Table 2).
| Variables/Time | Mean ± SD | Test Value | P-Value |
|---|---|---|---|
| Modified Ashworth Scale | 9.16 | 0.0001 | |
| Onset of treatment | 0.51 ± 3.46 | ||
| End of treatment | 1.46 ± 0.91 | ||
| Modified Tardieu Scale | 14.6 | 0.0001 | |
| Onset of treatment | 3.6 ± 0.5 | ||
| End of treatment | 1.26 ± 0.7 | ||
| Spasm Frequency Score | 12.38 | 0.0001 | |
| Onset of treatment | 3.88 ± 13.13 | ||
| End of treatment | 2.21 ± 3.8 |
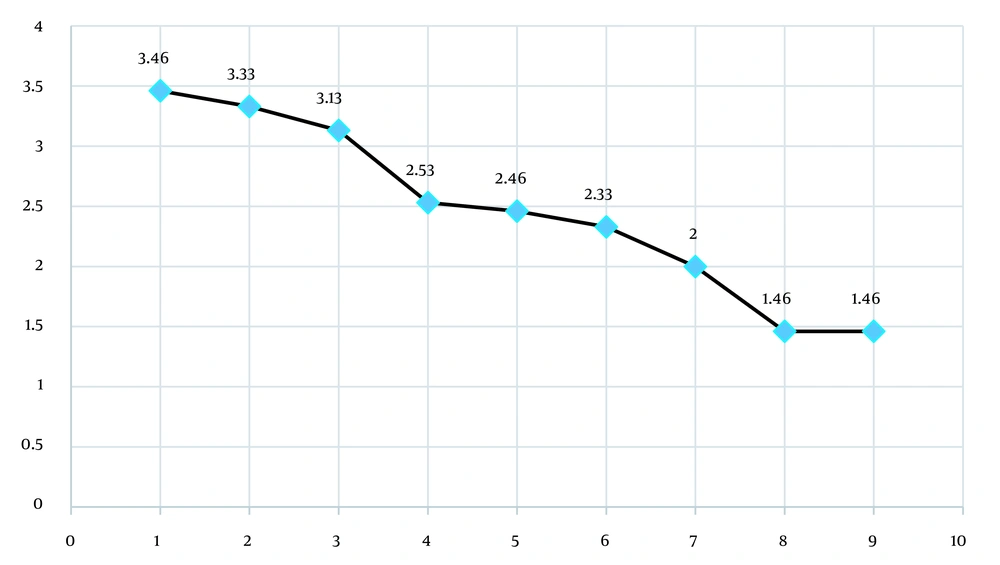
Repeated measures analysis of variance (ANOVA) showed that there was a significant difference in the mean scores of MAS at different times during treatment with intranasal lidocaine 0.5% in patients with TBI (P = 0.0001; Figure 1).
Paired t-test found that there was not any significant difference in the mean RASS values at the time of treatment initiation and end of treatment (P = 0.131), in the mean of GCS values at the time of treatment initiation and end of treatment (P = 0.677), in MAP at the time of treatment initiation and end of treatment (P = 0.717), in the mean values of SPO2 at the time of treatment initiation and end of treatment (day 9) (P = 0.255), in the mean values of HR at the time of treatment initiation and end of treatment (P = 0.074), and in the mean values of RR at the time of initiation of treatment and end of treatment (day 9) (P = 0.109; Table 3).
| Variables/Time | Mean ± SD | Test Value | P-Value |
|---|---|---|---|
| RASS | 1.6 | 0.131 | |
| Onset of treatment | -1.4 ± 1.45 | ||
| End of treatment | -0.93 ± 0.96 | ||
| GCS | 0.425 | 0.677 | |
| Onset of treatment | 10 ± 02 | ||
| End of treatment | 10 ± 02 | ||
| MAP | 0.37 | 0.717 | |
| Onset of treatment | 97.33 ± 12.8 | ||
| End of treatment | 95.73 ± 17.89 | ||
| SPO2 | 1.18 | 0.255 | |
| Onset of treatment | 97.53 ± 1.18 | ||
| End of treatment | 1.84 ± 98.13 | ||
| Heart rate | 1.93 | 0.074 | |
| Onset of treatment | 10.8 ± 93.6 | ||
| End of treatment | 17.15 ± 84.26 | ||
| Respiratory rate | 1.7 | 0.109 | |
| Onset of treatment | 3.8 ± 24.06 | ||
| End of treatment | 5.06 ± 26.4 |
Repeated measures ANOVA showed a significant difference in the mean scores of MTS at different times during treatment with intranasal lidocaine 0.5% in patients with TBI (P = 0.0001; Figure 1).
5. Discussion
After a brain injury caused by a stroke or trauma, patients typically experience impaired movement on one or both sides of the body. The cause of most motor and functional disorders in these patients is spastic hypertonia. This abnormal and excessive muscle tone can lead to problems such as pain, limitation in the movement of an extremity, interference with the ability to walk and perform daily activities such as dressing and stabilization of the limb in an unfavorable position (32, 33).
The researchers of this study found no similar studies in the literature. Hence, we compared our findings with some almost similar studies.
In this study, although all the patients were treated with the maximum dose of baclofen for 48 hours, the use of baclofen had no effect on the severity of spasticity in the MAS and MTS and on the frequency of spasticity in the SFS, and a significant decrease in spasticity was reported only in continuous intranasal infusion of lidocaine 0.5%. A study on a 34-year-old man found that the subcutaneous use of bupivacaine 75% was effective in treating spasticity of spinal origin that did not respond to conventional therapies and was also resistant to intrathecal baclofen (34).
In a study by Wang et al. on patients treated with ITB (intrathecal baclofen), it was found that treatment with intrathecal baclofen in all patients, except one, resulted in a significant reduction in scores of spasmodic severity, motor impairment, and functional disability (35). In the present study, the use of baclofen had no effect on reducing the severity of patients’ spasms. The difference between the present study and that of Wang et al. may be due to differences in the method of administration of baclofen. In the study by Wang et al., the effect of baclofen administration by implantable intrathecal pump was assessed 39.4 months after acquired brain injury (ABI), while in the present study, baclofen was administered orally at the hospital, and if remained untreated, lidocaine infusion was used for the patients.
Another research reported the effect of long-term intrathecal infusion of bupivacaine 0.5% in relieving pain and spasticity in a 56-year-old patient with multiple sclerosis (MS) who did not respond to analgesic therapies, baclofen, opioids, peripheral neurolysis (obturator and lumbar plexus nerves), and intrathecal neurolysis of the L4 - S3 nerve roots. The results showed that intrathecal infusion of bupivacaine 0.5% at a dose of 15 mg per day, which was gradually increased to 95 mg per day over 68 days, could completely relieve the patient’s pain and spasticity. However, it should be noted that this study was a case study performed only on one patient with MS (36).
Another study evaluated the effect of a single dose of intrathecal fentanyl and lidocaine in the treatment of 11 patients with spasticity due to spinal cord injury or disease. In this study, 50 mg of intrathecal lidocaine was first injected to each patient, and spasticity was assessed 30 minutes after injection. Then, 24 hours later, after the complete return of the initial spasticity, 35 μg of fentanyl was injected intrathecally, and the spasticity was assessed 90 minutes after the injection. With both lidocaine and fentanyl, spasticity was significantly reduced, and the passive range of motion was greatly increased (36). Although the procedure and method of using of lidocaine in this were different from our study, it seems that the use of lidocaine is effective in reducing spasticity due to spinal cord injury.
The main limitations of this study included the small sample size, the need for ICU admission of patients, and the inability to measure lidocaine concentrations in the serum and CSF. Therefore, it is recommended that further multi-center studies be performed with larger sample sizes due to the high probability of return of spasticity with discontinuation of treatment within longer periods, using higher doses, along with the measurement of blood and CSF levels of the drug to determine the therapeutic window and the maximum effective intranasal dose in patients with spasticity caused by TBI.
5.1. Conclusions
According to the findings of this study, continuous intranasal infusion of lidocaine appears to be effective in patients with spasticity caused by TBI. It is recommended that studies with larger sample sizes be performed. Also, study limitations such as the lack of blood level measurement of the drug for more accurate monitoring should be mentioned.