1. Background
Pain is one of the most prevalent and worrisome complaints of patients with cancer, particularly advanced or terminal cancer patients, with an approximate incidence of 67% (1). In recent years, interest in the treatment of cancer related-pain has been gaining attention due to catastrophic consequences on the quality of life (2). Due to the widespread adoption of the World Health Organization (WHO) “three-ladder” pain management in the 1980s, 80% - 90% of cancer patients can achieve satisfactory pain relief (3, 4). However, the remaining 10% - 20% of cancer patients do not attain adequate pain relief (5). In addition, serious side effects inevitably occur in patients along with the increased use of oral analgesic medications (6, 7). For these patients with refractory cancer pain or intolerable adverse events, some modalities (8, 9) and advanced pain management techniques are required to control pain, such as nerve blocks (10, 11) or intrathecal drug administration (5, 12, 13). Central neural blockade is an important modality for cancer pain management, but one should always be careful about its side effects (14-16). Although neuromodulation has a special role in the treatment of chronic refractory pain, its indications for cancer pain are limited (17-19).
The Intrathecal Drug Delivery system (IDDS) is applied in clinical settings, which provides better pain control with decreased opioid dosages compared to the traditional oral or parenteral routes (20, 21). The IDDS is effective within a limited range (22), leading to its fewer side effects as well as restricted analgesia to pain at different levels. The advanced IDDS device consists of a small, programmable pump that is implanted beneath the skin of the abdomen, and is attached to a catheter tunneled to the site of spinal entry, such as the Medtronic SynchroMed II Infusion system (Medtronic Inc., Minneapolis, MN, USA) (23). The programmable IDDS device provides patient-controlled analgesia (PCA) via adjustable, continuous intrathecal delivery of medication.
It is difficult and risky to implant the IDDS tip into the cisterna magna. There is only one report in five cases about its application in treating benign craniofacial and upper cervical pain with extremely encouraging results but no untoward effect (24). Besides, the analgesia for advanced intractable pain with the cisterna magna injection of phenol (25) or continuous infusion of bupivacaine (26) was investigated in previous trials. The PCA of cisterna magna morphine was investigated (27, 28) in western countries. However, its application to treat refractory cancer pain above the middle thoracic vertebrae level has not been fully investigated in China because of the heavy economic burden.
We, therefore, designed a clinical trial to investigate the efficacy and safety of this advanced technique for pain relief in Chinese advanced cancer patients suffering from refractory pain above the middle thoracic vertebrae level. We present the following review article focused on the role of cisterna magna morphine delivery as per the SPIRIT reporting checklist.
2. Methods
2.1. Patient and Public Involvement
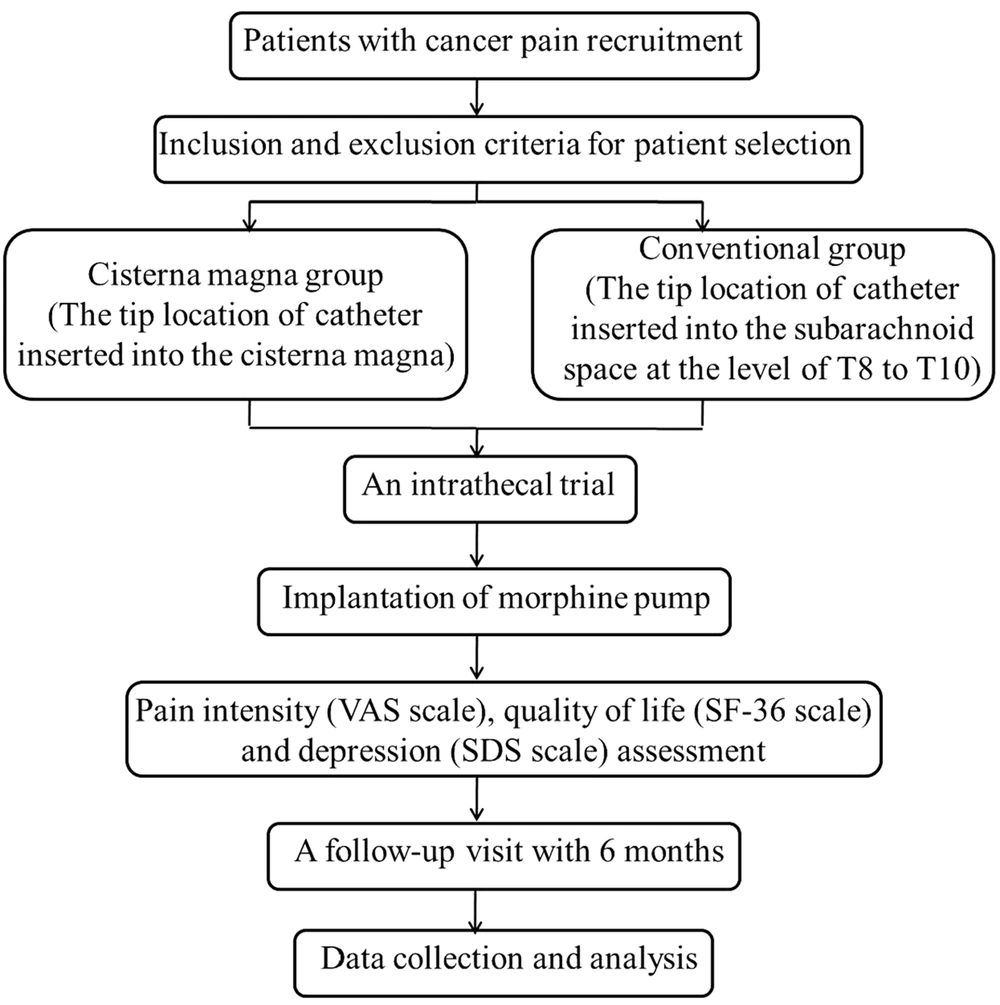
This is a prospective, non-randomized, multicenter clinical trial. Adult patients with refractory cancer pain will be prospectively recruited from the pain centers in two hospitals: The Chinese General Hospital of People’s Liberation Army (PLA) and the First Affiliated Hospital of China Medical University. The trial is conducted according to the principles of the Declaration of Helsinki (as revised in 2013). The trial protocol was approved by the Medical Ethics Committee of Chinese General Hospital of PLA and was registered in the Chinese Clinical Trial Registry (no.: ChiCTR-ONN-17010681, http://www.chictr.org.cn). Informed consent will be obtained from the patients or their close relatives before the trial. The scheme of the current protocol can be found in Figure 1.
2.2. Study Objective
The purpose of the current study, therefore, is to assess the analgesic effect of the cisterna IDDS for cancer patients suffering from refractory pain above the middle thoracic vertebrae level. Meanwhile, the quality of life and depression, as well as its safety documented with potential complications, will be the secondary outcomes.
2.3. Study Population
The patients will be included according to the following criteria: (1) The pain score on the Visual Analogue scale (VAS) persistently > 5; (2) inadequate analgesia (unsatisfactory pain relief at a dose > 400 mg of oral morphine or the equivalent); (3) intolerable drug toxicity with oral administration; (4) refractory pain above the middle thoracic vertebrae level; and (5) a successful morphine trial.
Participants will be excluded according to the following criteria: (1) Allergy or sensitivity to morphine; (2) severe coagulopathies, requiring anticoagulation therapy; (3) intracranial hypertension, sepsis, or infection at the site of the catheter or pump insertion; (4) serious mental disorders; (5) other severe diseases; (6) unable or unwilling to have the pump refilled; and (7) drug abuse.
Eligible patients will receive the implantation of the Medtronic SynchroMed II device at the cisterna magna. Baseline data will be collected, including demographic features, primary cancer, metastatic site, pain location, reasons for receiving the IDDS, and oral morphine equivalent doses.
2.4. Intrathecal Trial Protocol
To identify potential patients who are qualified for IDDS implantation, an intrathecal trial will be performed before the main operation (29). All opioids will be ceased for at least 12 hours before the trial. A spinal needle will be placed into the cisterna magna space with computed tomography (CT) guidance. Next, sterile saline containing morphine will be immediately injected into the cisterna magna region. Generally, the ratio of an intrathecal dose to an oral dose is 1:300 (30). Each intrathecal trial will be implemented with single bolus injection. A successful trial is defined as > 50% pain relief, with few or no severe adverse events. Patients with a successful intrathecal trial result will continue to the IDDS implantation.
2.5. Cisterna IDDS Implantation
The cisterna IDDS operation will be carried out in a sterile CT room. Patients will be placed in the lateral position with their heads bent slightly forward. A small vertical incision will be cut in the posterior cervical midline approximately two inches below the inion. A 15-gauge spinal needle will be inserted into the middle of the cisterna magna space under CT guidance. After a free flow of cerebrospinal fluid (CSF) is confirmed, the intrathecal catheter will be inserted into the cisterna magna through the needle lumen, still under CT guidance. The spinal needle will then be carefully removed. The intraspinal catheter will be fixed at the position using an anchor to prevent catheter movement induced by neck mobility. A subcutaneous pocket with a depth of < 2.5 cm will be created in the abdomen and used as the pump reservoir. The catheter will be tunneled subcutaneously to the abdominal wall and connected to the morphine pump, which will be filled with 400 mg morphine. Finally, the skin incision will be closed using a sterile technique. During the surgical procedure, blood pressure, respiratory rate, and heart rate will be monitored.
To reduce adverse events, the intrathecal morphine administered into the cisterna magna will be started at a dosage of 0.1 - 0.5 mg/d till satisfying analgesia (pain VAS score ≤ 3) is reached. The optimal outpatient dosing will be chosen based on the individual demand to reach satisfactory pain relief.
2.6. Pain Intensity Assessment
Pain intensity will be evaluated using the VAS (a 0-10 continuous scale ranging from no pain to the worst pain imaginable). Successful pain relief is defined as a VAS score of ≤ 3 or 50% pain reduction in the present protocol.
2.7. Quality of Life and Depression Assessment
Patients’ quality of life will be evaluated using the 36-item short-form health survey (SF-36). The SF-36 consists of eight dimensions, including physical function, physical role, body pain, general health, vitality, social function, emotional role, and mental health. The severity of depression will be assessed using the Self-rating Depression scale (SDS).
2.8. Follow-up Visit
According to our experience, the median survival of advanced cancer pain patients is approximately six months; thus, in this protocol, after IDDS, there will be a long-term follow-up over six months for all participants. The follow-up visits will be terminated due to the removal of the morphine pump or patient death. Pain VAS scores will be assessed and recorded on days 1, 7, 30, 90, and 180 of treatment. The SF-36 scores will be recorded on postoperative days 7, 30, 90, and 180. The SDS scores will be evaluated on postoperative days 1 and 7.
2.9. Outcome Measures
The primary outcome of the current trial is the analgesic efficacy represented by the reduction of VAS scores, percentage pain relief, and follow-up VAS alterations. The secondary outcomes will be the quality of life and depression, as well as its safety documented with potential complications.
2.10. Statistical Analysis
Statistical analysis will be performed with SPSS statistics 20.0 software. The differences between the parameters at baseline or individual follow-up visits will be compared using the two-sample t-test or the χ2 test. A Wilcoxon signed-rank test will be performed to compare follow-up data with baseline data. A P-value < 0.05 will be defined as statistically significant.
3. Discussion
Refractory cancer pain above the middle thoracic vertebrae level of advanced cancer patients is a difficult problem to manage. This challenge may be primarily attributed to the inadequate pain relief offered by lumbar cistern IDDS because the catheter tip cannot reach above the middle thoracic vertebrae level due to the catheter length limit. If the present trial demonstrates that the cisterna IDDS significantly improves pain intensity in patients, it is promising to manage refractory cancer pain above the middle thoracic vertebrae level in China.
The cisterna IDDS demonstrated its advantages in treating benign craniofacial and upper cervical pain in a previous study (24). Its analgesic efficacy for refractory cancer pain above the middle thoracic vertebrae level has also been investigated in western countries (27, 28). However, its application to treat refractory cancer pain in China has not been introduced or investigated because of the heavy economic burden for advanced cancer pain patients. We hope the current investigation can offer some evidence for its future inclusion in the medical insurance system of China and elsewhere.
The principal worry regarding this new approach may be the safety owing to the invasive procedure in the brain. However, the cisterna magna is a relatively large space in the brain between the medulla oblongata and cerebellum, where it is more convenient to puncture compared to other cerebral regions above the cervical level (31). In addition, the catheter tip will be placed in the middle of the cisterna magna space under CT guidance, which will greatly reduce the risk of brain injury. Severe drug-related toxicities (32), such as constipation, nausea, and vomiting (33), are the most frequent complaints made by cancer pain patients that lead to the consideration of intrathecal morphine therapy. These morphine-associated side effects may be significantly decreased due to the much lower dose required for the intrathecal route compared to the oral route.
Previous studies have demonstrated that cancer pain, particularly intractable pain, plays a crucial role in reducing the patients’ quality of life (2, 34). In addition, the quality of life is an important factor in evaluating the influence of disease or intervention on the physiology, psychology, and social activity of patients. Besides the decreased quality of life, most terminal cancer patients with refractory pain also suffer from depression (2), which may impair their cognitive function and dampen the therapeutic effect of anti-cancer. Quality of life and depression may be improved following sufficient pain relief.