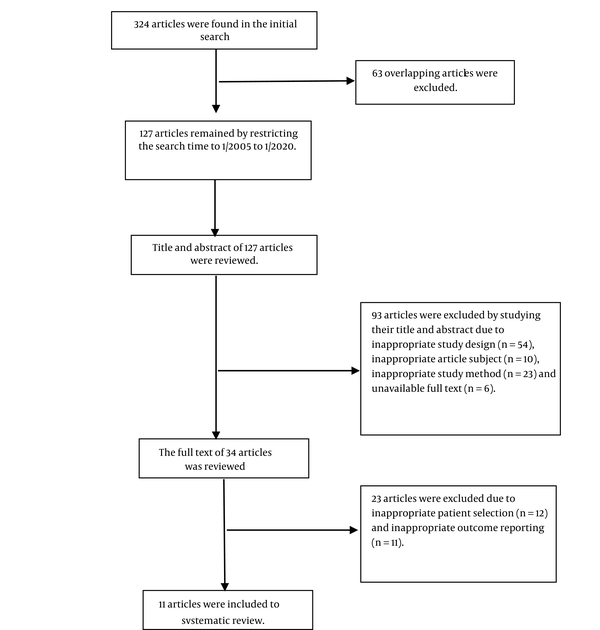1. Context
Proper cerebral oxygenation during anesthesia and resuscitation in severe brain injury is important in neurosurgery (1). The human brain comprises only 2% of total body weight but receives 20% of the body’s oxygen. It highlights the importance of sufficient energy and oxygen for the optimal health and function of the brain, and that the brain tissue is highly sensitive to the absence of blood supply and lack of oxygen, which can cause irreversible damage (2, 3). Decreased cerebral oxygenation during surgery is associated with cognitive impairments, high levels of stroke or coma, increased postoperative non-neurologic complications, and prolonged hospital stay (4, 5).
There are several ways to evaluate cerebral oxygenation. One of the most common ways to monitor the oxygen supply to the brain is by controlling the oxygen saturation in the jugular vein (SjVO2). Readings from a catheter inserted into the jugular vein outflow tract allow a comparison between arterial and venous saturated oxygen and determine the balance between cerebral oxygen stores and oxygen demand. The unsaturation of jugular venous blood may represent the early stages of cerebral ischemia. The limiting factor of this method, in addition to being invasive, is that it represents the overall blood supply to the brain and does not provide specific information about the blood supply to the injured area (6-9). Another method to measure brain tissue oxygenation (PbtO2) is catheterization in cerebral white matter. The most common system is LICOX. The system includes a monitor to display the oxygen and temperature values and has cables connected to control probes in the brain. This is also an invasive procedure and is not possible to be used for patients affected by sedation or mild anesthesia (10-12).
Near-infrared spectroscopy (NIRS) is common for cerebral oxygenation monitoring. Regional cerebral oxygen saturation (rSO2), using the NIRS method, contributes to real-time scanning of oxygen delivery from the cerebral cortex. The NIRS is a noninvasive device able to monitor cerebral oxygenation in the surgery process and bed (13, 14). Cerebral NIRS is increasingly being used for the early detection of oxygen deficiency in the brain. This method works based on near-infrared light irradiation (700 to 850 nm) to penetrate the surface layers of the head, including the scalp and skull, and to receive and process reflected light (15). This device measures the concentration of oxyhemoglobin (HbO2) and deoxyhemoglobin (HbR) in non-pulse blood flow using near-infrared spectrum and shows the cerebral perfusion status using HbO2 and HbR absorption difference at wavelengths of 700 - 850 nm (15, 16). Many factors could interfere with the precision and outcome of rSO2 levels, including skull thickness, extracranial tissue saturation, cerebrospinal fluid volume, head position, anesthesia status, and device model (17, 18). Despite these limitations, the NIRS monitoring non-invasively shows rSO2 levels, adequacy of cerebral perfusion during different operations such as carotid endarterectomy (19), cardiac surgery (20), beach chair surgery (21), and neurosurgery (22, 23), and the early symptoms of cerebral ischemia, and can prevent neurological complications (24, 25).
2. Objectives
There have been several systematic reviews on various types of surgery. These studies have shown the positive role of this technology in preventing neurological complications (26-29). However, since there is limited information on the use of NIRS in neurosurgical patients, this systematic review was carried out to review studies investigating the use of NIRS in the prevention of neurological complications during neurosurgery and postoperative care.
3. Data Sources and Search Strategies
The present study is a systematic review of the predictive power of cerebral oximetry monitoring in patients undergoing neurosurgery. The study was conducted by reviewing the databases, including Embase, PubMed, Web of Science, Scopus, and Cochrane Library between January 2005 and January 2020. The articles were searched mainly using systematic searches and valid keywords of cerebral oximetry, neurosurgery, near-infrared spectroscopy, NIRS, and possible combinations. In addition, references of selected articles were screened for relevant studies.
The inclusion criteria were as follows: studies with adult patients undergoing neurosurgery and postoperative care; studies using NIRS technology to evaluate the cerebral oxygenation status of patients to assess the effectiveness of NIRS on nervous system health; and studies investigating the effectiveness of NIRS technology on cerebral oximetry monitoring. The exclusion criteria were: (1) descriptive and qualitative studies; (2) studies not using NIRS to evaluate the outcomes of ischemia/hypoxia; (3) studies not published in English language; (4) and studies on patients under 18 years of age.
The selected studies had been published from January 2005 to January 2020. This period was chosen to reduce the effect of time, which might happen through the changes in medical facilities and advances such as non-invasive monitoring systems like NIRS and neurosurgical procedures and subsequent care.
4. Study Selection and Data Extraction
Initially, a list of titles and abstracts of all the articles on the searched databases were provided by two researchers and were independently reviewed to detect and select relevant titles. Subsequently, the related articles were independently included in the research process. Any disagreement between the two researchers in selecting specific articles was judged by a third party.
The initial search with no time restriction resulted in the retrieval of a total of 324 articles (Figure 1). After eliminating duplicate articles and limiting the search to the desired time interval, 127 articles with appropriate potential were obtained, whose title and abstract were evaluated based on inclusion and exclusion criteria. Of these, 93 articles were excluded and the full texts of 34 articles were reviewed based on inclusion and exclusion criteria by the researchers. Finally, 11 studies were selected for systematic review. The selected articles were thoroughly reviewed, and their information was recorded in a data extraction form. The data extracted from these articles included study objectives, field of study, method of sampling, inclusion and exclusion criteria, sample size, reference diagnostic methods to confirm ischemic/hypoxic brain injury, standard reference of monitoring cerebral oxygenation, study ethics, and discussions on the complications of impaired cerebral oxygenation during neurosurgery.
5. Results
After eliminating duplicate and unrelated studies, 11 articles were selected and reviewed (Figure 1). Meta-analysis was not possible due to high heterogeneity in neurological and neurosurgical conditions of patients, expression of different clinical outcomes, and different standard reference tests in the studies reviewed. Out of 11 articles included in this systematic review, seven studies were prospective cohort studies, three were retrospective cohort studies, and one was randomized controlled trial (Appendix).
5.1. Cerebral Oximetry with NIRS Technology in Spinal Neurosurgery
In a randomized controlled trial, Trafidło et al. at Lodz University of Medical Sciences, Poland, investigated the diagnostic value of using NIRS in major neuro spinal techniques to reduce postoperative cognitive dysfunction (30). In this study, 43 patients were assigned to group 1 (n = 13) using NIRS (INVOS 5100, Somanetics Corporation, USA) and group 2 (n = 30) using NIRS blind control. If the rSO2 decreased by 20% from baseline, mean arterial pressure (MAP), heart rate (HR), and systemic pulse oximetry saturation (SpO2) adjustments were made until rSO2 level reached baseline. To evaluate postoperative disorders, the researchers performed pre- and post-operative psychiatric tests. The first group, which was controlled by NIRS during the operation, performed better in the tests one week and one month after surgery (P < 0.05). Shortcomings of this study included a small sample size in the NIRS-controlled group, not expressing the mean and dispersion of other neuropsychological tests except Mann-Whitney test results (to compare postoperative cognitive disorders), and not expressing the magnitude of the difference in postoperative cognitive dysfunction (30).
Murniece et al. performed a prospective study through spine surgery to investigate the use of NIRS and to find its effect on postoperative cognitive function (31). This study was conducted on 34 patients in two groups of study (n = 23) with NIRS-based monitoring (INVOS 4100) and control (n = 11) monitored blindly. In the study group, the NIRS-based algorithm would be initiated if the rScO2 reduced bilaterally or unilaterally up to over 20% from baseline levels, or under an absolute level of 50%. Montreal-Cognitive Assessment (MoCA) scale was used to investigate cognitive function. In this study, rScO2 declined under the threshold in three cases and the NIRS-based algorithm was active. Despite significant changes in MAP, SpO2, and EtCO2, RScO2 levels returned to above threshold following compensatory reactions (MAP, HR, and SpO2 regulations). None of the three patients had postoperative cognitive dysfunctions (POCD). Also, in placebo group, one case exhibited a 34% decrease in rScO2 and presented with a POCD. This study concluded that using the NIRS-based clinical algorithm prevented POCD in patients after spine neurosurgery (31).
Soh et al. performed a prospective study on 106 patients over 60 years to investigate the association between rSO2 variations and delirium after spinal surgery (32). The study was conducted in the period between October 2014 and October 2015 at Yonsei University Severance Hospital, Seoul, South Korea. The rSO2 level was measured before and during surgery using NIRS. According to the results, nine (8%) patients showed symptoms of delirium 48 hours after surgery. The median decrease in rSO2 < 80% in patients with delirium was not significantly different from those without delirium (55% versus 56, P = 0.876). In addition, the decrease of rSO2 < 80% from baseline and blood pressure changes did not correlate with each other. According to the findings of this study, continuous intra- and post-operative monitoring of rSO2 levels by NIRS could not provide data on the incidence of delirium in aged cases after spinal surgery. One of the weaknesses of this study was lower than expected delirium (8 versus 18%), which limited the statistical power of the study. In addition, the lack of postoperative NIRS measurement could also be a confounding factor in the final conclusions (32).
5.2. Cerebral Oximetry with NIRS Technology in Brain Surgery
In a prospective study, Calderon-Arnulphi et al. at the University of Illinois investigated the usage of NIRS in cerebral ischemia monitoring over neurovascular procedures opening (33). They studied this procedure for 25 neurovascular cases, including clipping of aneurysm, resection of arteriovenous malformation (AVM), carotid endarterectomy (CEA), superficial temporal artery–middle cerebral artery (STA-MCA), external carotid artery–middle cerebral artery (ECA-MCA) bypass, EDMS, and balloon occlusion testing (BOT), to study cerebral oxygenation. Intraoperatively, quantitative frequency-domain NIRS (Q-NIRS), oxyhemoglobin (HbO2), deoxyhemoglobin (HHb), total hemoglobin (THb), and oxygen saturation in brain tissue (SO2) were measured as evidence of cerebral ischemia. Five patients showed clinical ischemia during surgery, all of whom exhibited monitoring of Q-NIRS, a lower HbO2, THb, and SO2, and a rise up in HHb in the affected area. In this study, the researchers did not use the standard reference test but compared the measurements with the opposite hemisphere. In addition, as the results of quantitative nature of Q-NIRS methods, recordings were comparable with time, as well as recordings obtained in other patients. The findings of this study supported the role of NIRS as a non-invasive and continuous method for assessing intraoperative cerebral oxygenation (33).
In a prospective study at Jichi Medical University in Tokyo, Ebihara et al. investigated cerebral ischemia using NIRS with oxygen inhalation (34). In this study, 30 volunteers with no history or background of cerebral disease were compared with 33 cases with cerebral ischemia using NIRS (TEG-4000, Hitachi) with oxygen aspiration. The patients with ischemia or obstruction were diagnosed with either internal carotid artery (ICA) or middle cerebral artery (MCA) through magnetic resonance (MR) angiography. In these subjects, cerebral blood flow (CBF) variations in MCA were evaluated by N-isopropyl-p- [123I] Iodoamphetamine (123I-IMP) SPECT, as the standard reference test with NIRS. In normal volunteers, there was no evidence of decreased tissue oxygenation in 83% (25 out of 30), whereas the NIRS in all ischemic patients showed less oxygen supply in ischemic regions than in normal regions. In 85% (28 of 33 cases) with cerebral ischemia, the NIRS findings were consistent with SPECT findings. These results indicated that NIRS could be used to evaluate cerebral oxygenation repeatedly and non-invasively (34).
In a prospective study, Bhatia et al. compared the NIRS data (Invos 4100, Somanetics) obtained during coil embolization with the prevalence of vasospasm detected by angiography (22). The subjects included 32 subarachnoid hemorrhages (SAH) patients undergoing embolization between February and November 2005 at King's College Hospital, London. Bilateral NIRS was fixed. Among these, 15 (46.9%) patients experienced spasms, two of whom were severe or totally. There was no significant proportion between aneurysm and baseline rSO2 with NIRS (P = 0.243). Although, there was a significant relationship between vasospasm and its degree (especially at severe and total levels) and reduced NIRS data on rSO2 (P < 0.001) (22).
In a prospective study, Shafer et al. evaluated the possible relationship between two NIRS diagnostic devices (INVOS-5100) and enhanced portable xenon CT (Xe/CT) (35). The study was conducted from June 2008 to December 2009 at the University of New Mexico Hospital and imaging studies were collected from 22 patients with SAH and traumatic brain injury (TBI). There was no statistically significant relationship between the two diagnostic tests. The values were compared using Spearman correlation coefficients, which were 0.05 and -0.05, right and left, respectively. The researchers mentioned several limitations, including the small sample size, various causes and sites of brain injury, and the differences in specific patient physiological factors (35).
Mazzeo et al. in a retrospective study measured rSO2 in 25 patients undergoing elective endovascular embolization of cerebral aneurysms, arteriovenous malformations, dural arteriovenous fistulas, and meningiomas under general anesthesia from January 2007 for a year (36). Continuous measurement of rSO2 was performed using NIRS (INVOS-5100 Somanetics Corp.) at the University of Messina in Italy. The rSO2 findings were significantly associated with different stages of the neuroendovascular procedure, including anesthesia induction, diagnostic angiography, intra-operative and post-anesthesia recovery (P < 0.001), with the most change being related to baseline rSO2 values during the intra-operative intervention. The effect of different cerebral pathology on rSO2 was known so that the more invasive the method, the greater the effect on rSO2. According to the results of this study, the NIRS was a potentially useful control tool during neuroradiologic procedures, which enabled the early detection of harmful intraoperative events resulting in cerebral hypoxia by evaluating rSO2 variations according to different stages of operation. Researchers also reported possible effects related to the use of contrast agents (36).
In a retrospective study, Taussky et al. examined the statistical association of NIRS and CT perfusion regarding CBF measurements (37). The study was conducted from February 2008 to June 2011, and included eight patients (six SAH patients, one ischemic stroke patient, and one ICH patient) admitted to Mayo Clinic in Florida. There was a linear relationship between frontal NIRS cerebral oxygenation measurements compared with regional CBF on CT perfusion imaging (P < 0.0001). The researchers also examined the advantages and disadvantages of various CBF measurement methods, such as the Kety-Schmidt model, PET, Xe/CT, and MRI w/perfusion-weighted imaging (37).
In a retrospective study, Matsumoto et al. investigated the efficacy of rSO2 monitoring by NIRS (INVOS-5100, Somanetics Corp.) during carotid artery stenting (CAS) to predict cerebral hyperperfusion syndrome (CHS) (38). The study was performed on 64 patients over one year from June 2006 to June 2007 at Kokura Memorial Hospital, Fukuoka. Pre-computed tomography (SPECT) and technetium-99m hexamethyl propylene amine oxime SPECT postoperatively were used as a reference method to evaluate cerebral perfusion. In the patients with postoperative CHS confirmed by SPECT, the range of rSO2 change was found > 24% versus < 10% (obtained by NIRS) in patients without CHS. This study showed that rSO2 measurement by NIRS could be a rapid and appropriate predictor of CHS after CAS (38).
In a prospective study, Vilke et al. examined the predictive value of NIRS for the survival rate of patients with TBI (39). The study was performed on 61 patients who underwent surgery for TBI from September 2011 to March 2013 at the Hospital of Lithuanian University. The rsO2 measurement by NIRS (INVOS series SOMANETICS spectrometers) continued until 72 hours after surgery. The survived patients showed higher levels of rsO2 at the periods of admission to an operation room (75.4 ± 9.8% versus 71.0 ± 20.5%, P = 0.013) and one hour after admission to intensive care unit (ICU) (74.7 ± 1.5% versus 61.9 ± 19.4%, P = 0.029). It was also found that one hour after admission to ICU, the rSO2 values did not exceed 68.0% in the left hemisphere and 68.3% in the right hemisphere, the risk of death was enhanced up to 17.7 times (P < 0.01) and 5.1 times (P < 0.05), respectively. Thus, rSO2 below 68% was considered as a serious risk threshold for death in patients with TBI. The disadvantage of this study was the failure to measure systemic hemodynamic status at the time of rSO2 assay, which could affect rSO2 levels (39).
6. Discussion
In this review study, we examined the predictive power of cerebral oximetry monitoring in patients undergoing neurosurgery. This study reviewed 11 articles investigating the monitoring role of NIRS in patients undergoing neurosurgery. There are numerous studies on the efficacy of NIRS in clinical practice (40-45). In addition, non-invasive NIRS-based cerebral monitoring is used today in a variety of clinical conditions. There is evidence that the NIRS may be effective in reducing neurologic injury during surgery, although this role has often been demonstrated in cardiovascular surgery and similar evidence is not widespread in neurosurgery. The use of NIRS has not been approved as a standard practice for monitoring cerebral oxygenation in medical centers worldwide. However, methods such as jugular bulb oximetry or microdialysis are less commonly employed (46).
One of the main objectives in neurosurgical anesthesia is the prevention of cerebral hypoxia secondary to neurological injury. Cerebral oximetry can help diagnose periods of cerebral hypoxemia and intervene to prevent secondary brain injury and its consequences. The NIRS is used for this purpose. However, the use of NIRS for monitoring cerebral oxygenation has some limitations. Although extensive studies have reported encouraging results regarding the use of NIRS in neuromonitoring in the diagnosis of cerebral ischemic/hypoxic events during surgery, the threshold and protocol used to define and treat cerebral tissue desaturation vary between studies and operations (2). The absence of a standard threshold approved by medical societies is one of the most important limitations of this method for cerebral oxygenation. In the patients with scalp injury or skull fracture due to head trauma and those undergoing recent craniotomy or cranioplasty, the NIRS is an inaccurate tool because these conditions can affect the balance of infrared signal absorption level between the sensor and the brain, which determines the NIRS value. This change may be due to the presence of air or blood between the myocu taneous flapskull, the skull of the dura, as well as the distance between the dura and the brain (47). In this situation, as the distance between the sensor and the brain tissue increases, the infrared depth of penetration is insufficient to reach the brain, resulting in higher inaccuracies. Since cerebral oximetry sensors are predominantly located on the forehead (except in bald ones), they are considered to be a regional monitor and monitor a relatively small area of the brain. As a result, likely, regional ischemia is not reported in areas far from the sensors. The results obtained from NIRS are more reliable under low-perfusion conditions or extensive ischemia of the head tissues. Low-perfusion conditions rarely occur in brain and neurosurgery and are common in neurosurgical patients with impaired brain perfusion, while a sufficient source is available for superficial tissues (48). Some results suggest that the cerebral NIRS can control the oxygenation to head tissues rather than the brain; this concept should help clinicians achieve the cerebral NIRS range as a parameter for monitoring cerebral oxygenation and perfusion (49).
Studies comparing NIRS with other methods of measuring cerebral oxygen show a correlation between rSO2 and cerebral tissue oxygen tension as measured intensively (23, 50), while the relationship between values measured with NIRS and jugular bulb oximetry is inconsistent and contradictory (51-55). The NIRS data are obtained from arterial, venous, and capillary blood examination in a small area of the cerebral cortex and are affected by the level of oxygen uptake, while jugular bulb oximetry only examines venous blood and shows whole-brain oxygen saturation (56). In contrast, the PbtO2 measures extracellular oxygen tension and reflects the balance between cerebral oxygen consumption and supply. Thus, it can be concluded that these techniques also provide complementary information on the process of cerebral oxygenation (57-60).
The NIRS is a non-invasive cerebral oximetry that provides rapid, continuous, and measurable information on cerebral oxygenation, and can be used in a variety of clinical settings in contrast to other conventional methods, such as jugular bulb oximetry and direct measurement of PbtO2. The NIRS has been used for over two decades to monitor intraoperative oxygenation and to measure and characterize the hemodynamic and metabolic response of brain activity.
Although various studies have documented the functional role of cerebral NIRS monitoring in neurosurgery, further large-scale research or randomized controlled trials are needed to validate cerebral NIRS monitoring in patients with a neurological condition. There is also insufficient evidence to support the theory that the NIRS can be a reliable tool for predicting mortality or cerebral ischemia events and its long-term complications. Extensive research is needed to determine the threshold or critical limit of rSO2 by NIRS for cerebral ischemia or desaturation, as well as the association of rSO2 size with mortality and clinical complications, such as cognitive, motor, or sensory impairment in neurosurgery. Technological advances in NIRS and common standards are also essential for reading the cerebral NIRS monitoring from different companies, similar to those used in pulse oximeters.