1. Introduction
Acute massive pulmonary embolism (PE) is a fatal disease with a different clinical presentation. It is life-threatening that treatment is administered properly so that recurrent thromboembolism and death can be prohibited (1). Pulmonary embolism is classified based on the degree of involvement of the pulmonary arteries by blood clots and the patient's cardiopulmonary condition (2). So, the best way to classify a pulmonary embolism is based on the patient's hemodynamic condition.
Risk factors for PE are immobility, congenital coagulation conditions such as factor V Leiden, bone fracture, a history of cancer or chemotherapy, being overweight or obese, smoking cigarettes, pregnancy, taking oral contraceptives pills or hormone replacement therapy, history of cerebrovascular accident (CVA), paralysis, chronic heart failure, or hypertension, recent injury or trauma to a vein, age over 60 years (1). Klinefelter syndrome (KS) is a genetic disorder that affects men. It is not usually diagnosed until puberty and occurs at about 1.5 per 1000 males. The karyotype of these patients is usually XXY (3).
An augmented incidence of thromboembolism (TE) was described in patients with KS, with a described occurrence of 0.1 - 0.2% in the general populace and up to 3.1% in sterile males. The etiology of VTE in KS is not well understood (4). The KS is more prone to the possibility of thromboembolic events in the form of deep vein thrombosis (DVT) or massive pulmonary emboli. Although the main mechanism of the prothrombotic event in patients with KS is not clear, adrenal insufficiency and chromosomal abnormalities can be the cause of its occurrence. These factors themselves can be because of the increased incidence of thromboembolism (4).
2. Case Presentation
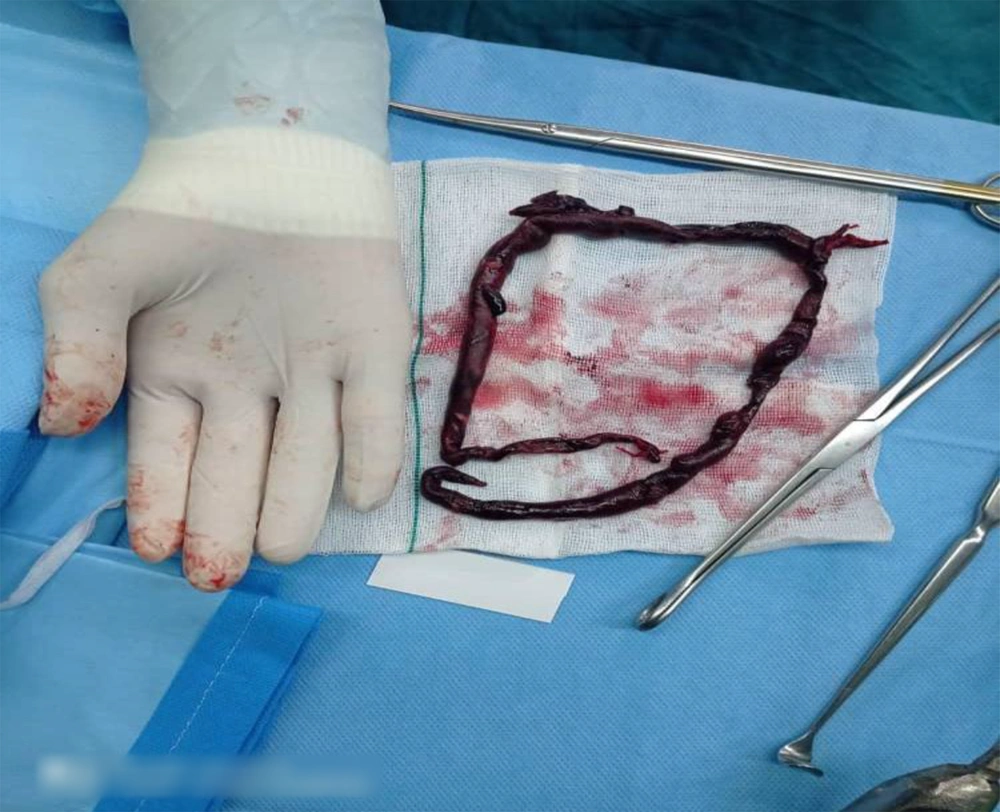
We introduced a 34-year-old man with a definitive diagnosis of KS from two years ago, with a history of trauma to the ankle from 18 days ago. His family history of venous thromboembolism (VTE) was negative. He was hospitalized in the cardiology ward to treat chest pain and dyspnea, with the New York Heart Association Classification of Heart Failure (NYHA) class III. The clinical examination at the time of admission in OR exposed a drowsy patient with a history of twice syncope from the day before, palpitation (PR = 120), sweating, chest pain, blood pressure at 80/55 mmHg (with invasive blood pressure monitoring IBP), SpO2at 85% in ambient air and 92% under oxygen, and two-sided crackles on chest auscultation. In paraclinical findings, a D-dimer test was 1700 mg/mL, ECG revealed tachycardia with RBBB, transthoracic echocardiography presented a D-shape septum due to high RV pressure, moderate to severe RV enlargement, moderate to severe RV systolic dysfunction, hypertrabeculated RV apex, at least moderate TR, TRG = 40 mmHg, severe PAH, PAP = 55 mmHg, dilated IVC with respiratory variation < 50%, visible fresh cloth in main PA, and proximal part of branches in suprasternal view. On computed tomography angiography (CTA) of the lungs, a massive embolus was reported in the main pulmonary artery as well as in the right and left main branches. The troponin was negative. The lower extremities venous Doppler ultrasound revealed normal flow and no thrombosis. Because of this massive pulmonary embolism, the patient was a candidate for surgical embolectomy. After general anesthesia and placement on the hypothermic cardiopulmonary bypass (CPB) in the 28-degree centigrade, pulmonary embolectomy was done (Figure 1). After rewarming, weaning off from the CPB was easily done, without the need for inotrope. After four hours, the patient was extubated and weaning off the ventilator with a stable hemodynamic condition. The congestive signs were retreated well using diuretic treatment. The patient was discharged from the hospital in good general condition after one week with a warfarin prescription.
3. Discussion
Although many advances in the diagnosis and treatment of pulmonary embolism, it is still associated with high mortality (5, 6). The etiology and pathogenesis of PE are multifactorial. Factors such as malignancy, operation, prolonged immobilization, fracture, paralysis, usage of oral contraceptives, and inherited coagulopathies are predisposing factors for PE (4, 7). In the patients with KS, the increased frequency of the VTE was frequently described. Its etiology is not well understood; nonetheless, it is supposed it can be due to opposite association among a synthesis of plasminogen activator inhibitor-1 (PAI-1) and levels of testosterone (8).
Tissue plasminogen activators are inhibited by PAI-1 produced by endothelial and adipose tissue, thereby decreasing the conversion of plasminogen to plasmin. Impaired function of fibrinolytic causes pro-thrombotic conditions (8). The role of high estrogen is higher than that of low androgen in causing thromboembolic events in KS. Estrogen destroys the amount of procoagulant and anticoagulant, especially in hereditary thrombophilia patients (9). As can be seen from the literature, the association between KS and thrombosis is due to the presence of antibody antiphospholipid (10). This antibody is caused by the factor V Leiden mutation (10). The vascular abnormalities, deficiency of the protein C and S, high levels of the homocysteine, and deficiency of the antithrombin III were considered the core pathogenesis of thromboembolism in patients with KS (11). The incidence of MI is higher in these patients (11). The KS cases were prone to recurrent VTE (12). Further studies are needed to understand the pathogenesis of pulmonary emboli disease in patients with KS. So, oral anticoagulant therapy should be recommended to patients with KS who have experienced thromboembolic events in their lifetime.