1. Background
The risk of mortality and morbidity associated with cardiovascular surgery is generally assessed using prognostic tools such as the European System for Cardiac Operative Risk Evaluation II (EuroSCORE II) and JAPAN scores (1, 2). These scores are calculated based on preoperative data such as age, sex, type of operation, medications, and past medical history. However, they do not include functional status, such as frailty and sarcopenia. Further, it has been reported that the EuroSCORE II cannot predict postoperative quality of life (QOL) (3). In addition, postoperative QOL may be overestimated in elderly patients who underwent cardiovascular surgery with a low preoperative QOL (4). Determining the surgical eligibility of elderly individuals is often challenging because their physiological tolerance to surgical stress varies widely, with some patients becoming bedridden or having poor functional outcomes. Moreover, age itself has been reported to be an important prognostic factor associated with postoperative cognitive dysfunction, including after cardiac surgery (5, 6), which affects patients’ daily activity. Therefore, the prognostic scores calculated using the currently used methods may not be applicable to elderly patients, especially those undergoing cardiovascular surgery (7).
Frailty increases vulnerability to stressors (8) and is associated with intensive care unit (ICU)-acquired weakness, nursing home admission, mortality, and morbidity (9-12). Sarcopenia, a major cause of frailty (13), is defined as aging-related muscle loss and it can adversely affect QOL (14). In patients undergoing cardiovascular surgery, sarcopenia is commonly assessed using the psoas muscle index (PMI), which is calculated by dividing the cross-sectional width of the bilateral psoas muscle at the level of the L3 vertebra by the square of the patient’s height (15). Previous studies have suggested a relationship between mortality and PMI after cardiovascular surgery, as well as several other illnesses (16, 17). However, these studies did not assess postoperative QOL, including the rate of discharge to home as a major outcome. Therefore, the usefulness of the PMI for determining the risk of poor postoperative QOL in patients undergoing heart surgery is still unclear.
2. Objectives
The present study aimed to evaluate the association between PMI and the discharge to home as an index of postoperative QOL after cardiovascular surgery in elderly patients. We hypothesized that a low PMI is associated with a lower rate of discharge to home in elderly patients undergoing cardiovascular surgery.
3. Methods
3.1. Study Design and Patient Population
This was a nested case-control study of patients aged ≥ 75 years who underwent elective cardiovascular surgery and were admitted to the ICU at Saitama Medical Center, Jichi Medical University between January 1, 2016 and March 31, 2017. Patients who underwent cardiovascular operations, including valve replacement, valvuloplasty, coronary artery bypass graft (CABG), and thoracic aortic surgery were included. The exclusion criteria were emergency cardiovascular surgery, transcatheter aortic valve replacement, abdominal aortic replacement, peripheral arterial surgery, and aortic stent-grafting. Patients without PMI data calculated using computed tomography (CT) scans were also excluded.
The study protocol was approved by the appropriate institutional review board. The need for written consent was waived owing to the retrospective nature of the study.
3.2. Nested Case-Control Design
We used a nested case-control design to evaluate the association of factors with the rate of discharge to home, considered one of the most accurate measures to evaluate intensive care outcomes in the elderly (18). The case group was defined as the poor outcome group and included patients who either died or were transferred to a rehabilitation facility postoperatively. The control group was defined as the good outcome group and included patients who were discharged home postoperatively.
3.3. Data Collection
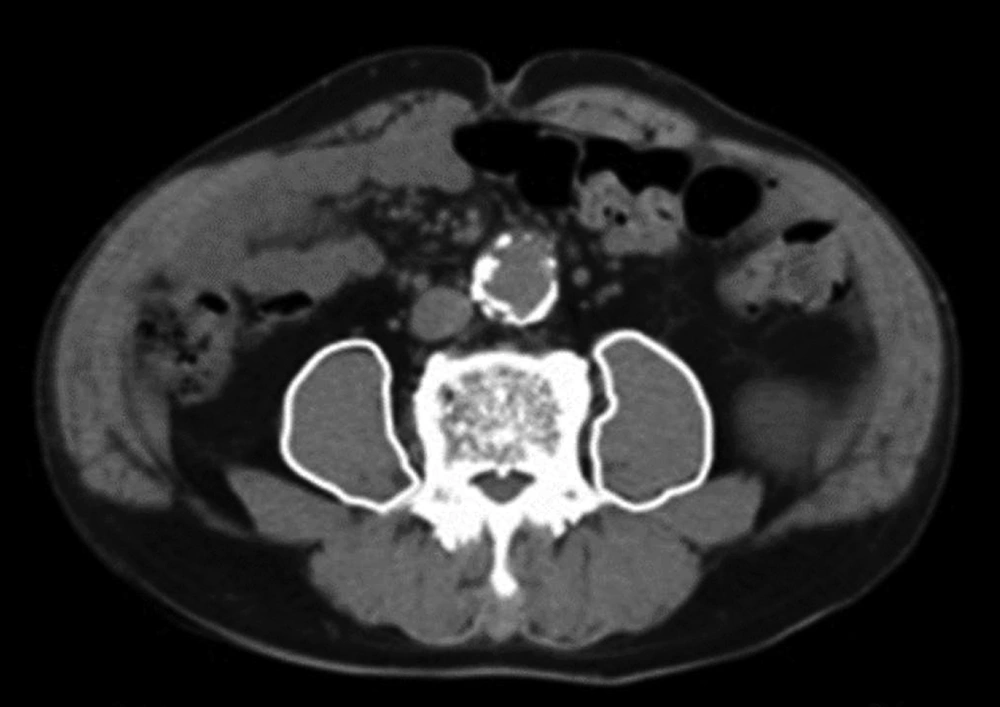
Patient data including sex, body weight, height, body mass index (BMI), medical history, updated Charlson comorbidity index (CCI) (19), primary disease, type of operation, EuroSCORE II, Acute Physiology and Chronic Health Evaluation II (APACHE II) score, and PMI were collected from hospital records. We used the updated CCI because Japanese data were used to validate the score, and it was shown to have a more linearly increasing relationship with mortality than the original CCI (19). PMI scores were calculated by the primary investigator via the manual trace method using CT scan images (Figure 1). At our institution, cardiovascular surgeons routinely obtain a thoracoabdominal CT scan of each patient within 3 months prior to surgery, and we were able to use data from these scans to calculate the PMI.
3.4. Statistical Analysis
Categorical variables were reported as either numbers or percentages, while continuous variables were reported as medians with interquartile ranges or mean values with standard deviation. The collected data were assessed to determine distribution patterns. Normality was compared using the Shapiro-Wilk test. Subsequently, an F-test was used to demonstrate equal variance. Normally distributed data were assessed using Student’s t-test, while non-normally distributed data were assessed using the Mann-Whitney U-test. Differences in categorical variables were compared using Fisher’s exact tests.
Associations between discharge to home and PMI, APACHE II score, updated CCI, EuroSCORE II, and operating time were assessed. These factors were selected to consider their clinical impact on outcomes after cardiac surgery. Logistic regression analyses were used to determine the odds ratios and 95% confidence intervals (CIs). Receiver operating characteristic (ROC) curves were plotted, and the area under the ROC curves for each factor was compared. All statistical analyses were performed using R software (The R Foundation for Statistical Computing, Vienna, Austria, version 3.5.2). All P-values were two-sided, and P ≤ 0.05 was considered significant.
4. Results
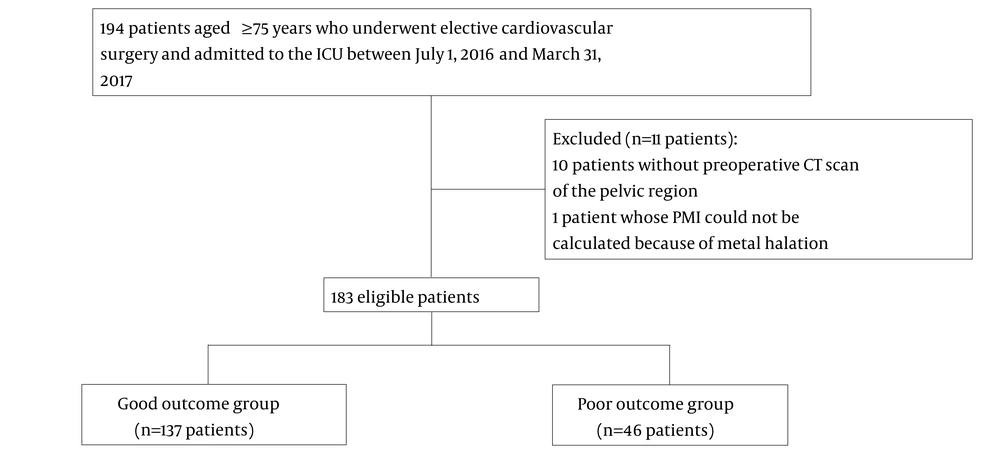
Of the 194 patients initially evaluated, 10 patients who did not undergo preoperative CT scans of the pelvic region and 1 patient who underwent lumbar fixation that prevented the calculation of the PMI were excluded. Finally, 183 patients were enrolled in this study. Among them, 137 and 46 patients were included in the good outcome and poor outcome groups, respectively (Figure 2). The patient characteristics are shown in Table 1. There were no significant between-group differences in the sex, age, and updated CCI. The median BMI was also not significantly different. Meanwhile, the mean height and weight, median APACHE II score, median PMI, and median EuroSCORE II scores were significantly different between the two groups.
| Good Outcome Group (n = 137) | Poor Outcome Group (n = 46) | P Value | |
|---|---|---|---|
| Sex, male (%) | 75 (55) | 18 (39) | 0.09 |
| Age (y) | 78 (76 – 80) | 78 (77 – 82.8) | 0.07 |
| Height (cm) | 156.1 (149.5 – 164) | 150.4 (145.9 – 160.3) | 0.03 |
| Weight (kg) | 56.0 (10.7) | 51.9 (11.0) | 0.03 |
| Body mass index | 22.6 (21.0 – 24.4) | 22.1 (19.7 – 24.4) | 0.25 |
| Updated CCI | 3 (2 – 3) | 3 (2 – 3) | 0.22 |
| Dementia (%) | 28 (20) | 20 (43) | < 0.01 |
| Hemodialysis (%) | 8 (6) | 3 (7) | 1 |
| Cancer (%) | 11 (8) | 2 (4) | 0.52 |
| Diabetes (%) | 36 (26) | 14 (30) | 0.57 |
| Hypertension (%) | 94 (69) | 26 (57) | 0.15 |
| Stroke (%) | 18 (13) | 7 (15) | 0.80 |
| EuroSCORE II | 3.49 (2.05 – 5.39) | 6.23 (3.2 – 10.59) | < 0.01 |
| Operation time | 284 (233 – 366) | 327 (262.5 – 428.5) | 0.01 |
| APACHE II | 16 (14 – 18) | 18 (16 – 21) | < 0.01 |
| PMI | 4.2 (0.96) | 3.3 (0.97) | < 0.01 |
| Type of operation | |||
| Valve replacement (%) | 98 (72) | 27 (59) | 0.14 |
| CABG (%) | 36 (21) | 13 (28) | 0.85 |
| Aortic surgery (%) | 26 (19) | 10 (22) | 0.67 |
| Other (%) | 4 (2) | 1 (2) | 1 |
Abbreviations: PMI, psoas muscle index; APACHE II, Acute Physiology and Chronic Health Evaluation II score; CCI, Charlson comorbidity index; EuroSCORE II, European System for Cardiac Operative Risk Evaluation II
aValues are presented as the median (interquartile range), mean (standard deviation), or number (%).
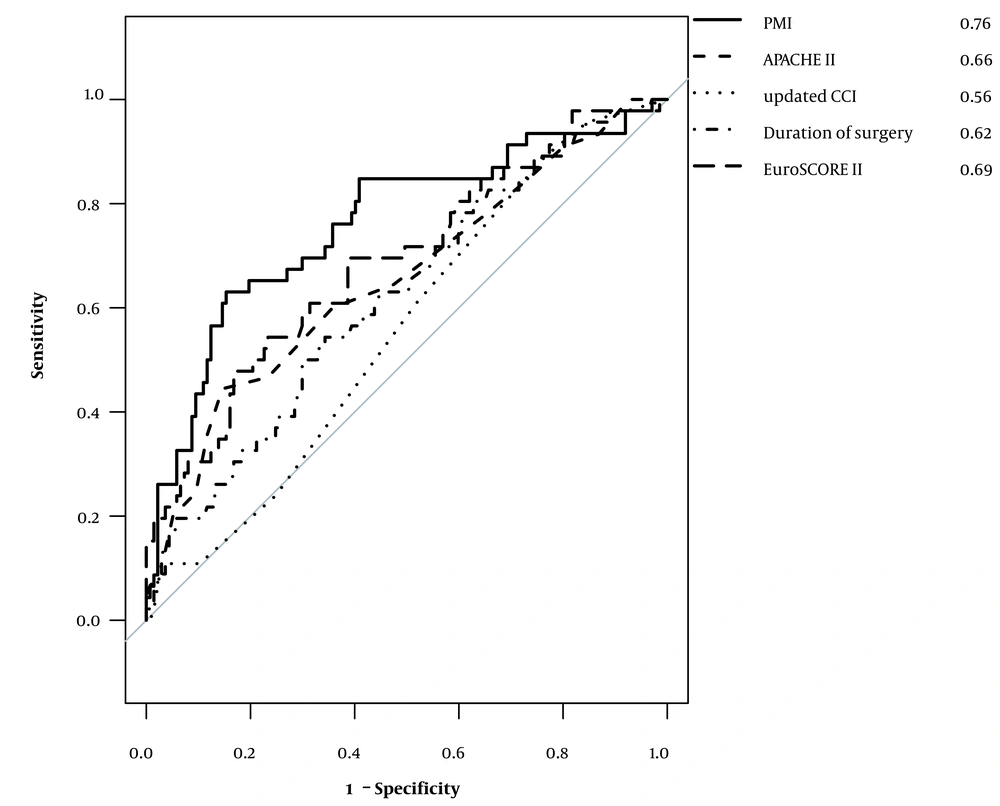
The results of the logistic regression analysis are shown in Table 2. PMI was significantly associated with the outcome at discharge, with an odds ratio of 0.25 (95% CI: 0.14 – 0.43; P < 0.01), which means that a common odds ratio for every one-unit increase in PMI for a poor outcome is 0.25. The ROC curves determined for each factor are shown in Figure 3. Comparison of the area under the curve (AUC) of the ROC for each of the assessed factors showed that PMI had the greatest AUC (AUC: 0.76; 95% CI: 0.67 - 0.85). A PMI cut-off of 3.24 had a specificity, sensitivity, positive predictive value, and negative predictive value of 0.86, 0.63, 0.58, and 0.87, respectively.
| Factor | Odds Ratio | 95% CI | P Value |
|---|---|---|---|
| APACHE II | 1.17 | 0.02 – 4.38 | 0.36 |
| EuroSCORE II | 1.13 | 1.02 – 1.26 | 0.014 |
| Operation time | 1 | 0.99 – 1.01 | 0.10 |
| PMI | 0.25 | 0.14 – 0.43 | < 0.01 |
| Updated CCI | 1.3 | 0.99 – 1.71 | 0.06 |
Abbreviations: APACHE II: Acute Physiology and Chronic Health Evaluation II score, EuroSCORE, EURO score II: European System for Cardiac Operative Risk Evaluation II; PMI, psoas muscle index; CCI, Charlson comorbidity index.
5. Discussion
This study shows that PMI is strongly associated with discharge to home in patients aged ≥ 75 years who underwent cardiovascular surgery. An increase in PMI was associated with a decrease in the odds of a poor outcome (defined as in-hospital death or discharge to a long-term care facility) adjusted by EuroSCORE II, APACHE II, operation time, and the updated CCI for patients aged ≥ 75 years who underwent cardiovascular surgery.
The EuroSCORE II and JAPAN scores were developed to predict postoperative outcomes and determine the appropriateness of cardiovascular surgery. However, these scoring systems do not reflect the influence of frailty, and only predict mortality and complication rates. These scores do not always predict other important outcomes for elderly patients, such as QOL after discharge and the rate of discharge to home. This study showed that PMI was more strongly associated with discharge to home than other clinical scoring systems associated with mortality following cardiovascular surgery.
Several studies have assessed the relationship between PMI and patient prognosis. Associations between poor prognosis and sarcopenia in patients with malignancies (20, 21) and those with traumatic injuries (22) have been reported. An association between mortality and PMI has been reported in patients who underwent cardiovascular surgery. Heberton et al. revealed the usefulness of PMI in predicting mortality in patients undergoing left ventricular assist device implantation (16). Saji et al. also reported an association between PMI and mortality in patients who underwent transcatheter aortic valve replacement (17). Okamura et al. reported that PMI was associated with long-term outcomes in patients who underwent cardiovascular surgery (23). Sarcopenia and frailty can prolong hospital stay and increase the need for transfer to rehabilitation facilities, both of which negatively affect patients’ QOL (24-26).
However, previous studies had important limitations. Some studies have assessed the impact of PMI by dividing the study cohort into two groups. They defined a sarcopenia group as patients below the fiftieth percentile of PMI values and the non-sarcopenia group as those above the fiftieth percentile, which includes the same number of patients (16, 21, 22). There was no justification for dividing patents by PMI to define them as sarcopenic or non-sarcopenic. In the present study, PMI was assessed as a continuous variable. Previous studies also included relatively young patients (approximately 65 years old). Sarcopenia and frailty are serious problems typically affecting the elderly who need intensive care (25), and studies of patients aged older than 75 years should be performed.
Further, previous studies used mortality as the primary outcome. Although mortality is an important outcome, we believe that the QOL of elderly patients is as important as mortality because a postoperative low QOL will result in high medical costs and a large care burden for families. Discharge to a care facility has been reported to be an accurate measure of intensive care and QOL outcomes in elderly patients (19, 27). Therefore, we evaluated the impact of PMI on QOL after cardiovascular surgery with discharge to home as the outcome measure. Our findings indicate that preoperative assessment of PMI may be useful in determining the indications for elective cardiac surgery in patients aged ≥75 years. Importantly, the findings indicate that a new prediction score that includes PMI or another frailty score is needed to assess surgical risk in the elderly.
This study has some limitations. First, it was not possible to adjust for confounding factors because of the retrospective nature of the study. Especially, preoperative QOL scores such as the clinical frailty scale could not be determined (8, 28). However, there is no consensus on the standard measurement of frailty (29). Sarcopenia is a major cause of frailty (13) and could be a surrogate marker of frailty. In this study, sarcopenia was evaluated using PMI, which is an objective indicator. One study used both PMI and intramuscular adipose tissue content to assess sarcopenia (26). A combination of these radiological parameters might assess sarcopenia more meaningfully, although there are few studies on intramuscular adipose tissue content. Second, this study included patients aged ≥ 75 years; therefore, the study had selection bias, and this may limit the generalizability of the findings. Third, the small size of the cohort also limited the number of factors that could be included in the conditional logistic regression analyses. Fourth, this was a single-center study, and the postoperative rehabilitation method was not adequately assessed. Some institutes utilize the active cyclic breathing technique for post-CABG patients (30). Such differences in rehabilitation programs may affect the patients’ postoperative outcomes, and thus the external validity of this study may be compromised. Finally, the long-term survival of the patients is unknown, including changes in living situations (for instance, moving between home and a rehabilitation facility). Discharge to a nursing home or rehabilitation facility following surgery does not always indicate a poor QOL. Some patients may eventually go home after rehabilitation. Therefore, a more complete follow-up evaluation is needed to determine QOL outcomes.
5.1. Conclusions
Compared to other established prognostic scores, PMI is more strongly associated with discharge to home in patients aged ≥ 75 years who undergo elective cardiovascular surgery. PMI may be helpful in determining the suitability of cardiovascular surgery in elderly patients.