1. Background
Pain after thoracotomy is often very severe and can be associated with severe complications such as atelectasis (1). Due to the retention of pulmonary secretions caused by pain during chest movements, this pain can also lead to severe pneumonia (2-4). The pain prevents effective coughing, deep breathing, and mobility of the patient. In general, severe postoperative pain increases postoperative complications and may also lead to chronic pain (5-7). There are many different ways to control acute pain after thoracic surgery. These treatments include systemic opioid-based treatment regimens, intercostal nerve block (ICB), newer methods of fascial plate blocking including serratus anterior plane block (SAPB) and pectoral nerves II block (Pecs II), thoracic paravertebral block (8), erector spinae plane block (9), and thoracic epidural block. There is considerable evidence that ICBs and fascial plate blocks are superior to systemic opioid-based regimens (10).
The intercostal nerve block is an effective method of post-thoracotomy pain relief that can be done with the help of anatomic landmarks, ultrasound, or fluoroscopy (8, 11-13). Unlike the use of systemic opioids that suppress the central nervous system and respiration and cause nausea and suppression of the cough reflex, ICB does not suppress the central nervous system. It is also relatively easy to perform and provides segmental analgesia when used at the desired dermatomal level (10). The use of ultrasound in guiding the block can significantly reduce the time and amount of medication use, as well as the risk of complications for the patient (14-17). Dexmedetomidine, an alpha-2 receptor agonist, is a drug with strong analgesic effects that appears to have synergistic effects with opioids due to opioid-sparing effects (18, 19). It has been shown to prolong analgesic effects of peripheral nerve and neuraxial blocks, with hypotension and bradycardia as its side effects (20-26). Ropivacaine is a long-acting topical analgesic with low-fat solubility, cardiac and central nervous system toxicity, and potency similar to bupivacaine (27).
2. Objectives
This study aimed to evaluate the effect of the intercostal nerve block with ropivacaine alone or in combination with dexmedetomidine for reducing postoperative pain after thoracotomy.
3. Methods
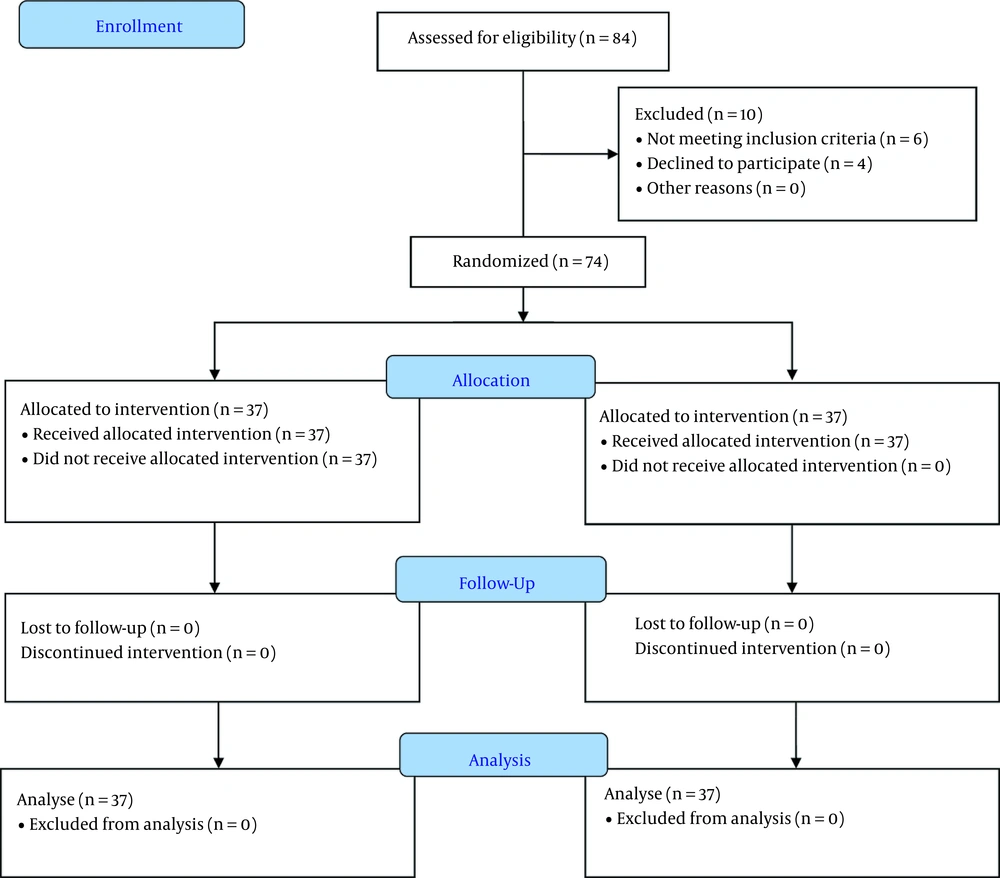
This study was a randomized clinical trial performed on 74 patients aged 18 to 60 years referred to a medical-educational center in Ahvaz, Khuzestan, Iran, between July 28, 2019, and February 3, 2021. The protocol of this study was reviewed and approved by the Ethics Committee of Ahvaz Jundishapur University of Medical Sciences (IR.AJUMS.HGOLESTAN.REC.1398.041) and was registered on the Iranian Registry of Clinical Trials (IRCT20190107042264N2). This study followed the CONSORT 2010 statement (Figure 1). Inclusion criteria included ASA class I or II, BMI less than 40, no history of substance or alcohol abuse, no pregnancy or breastfeeding, no history of coagulation problems or use of anticoagulants, ejection fraction greater than 30%, no history of cardiac blocks, no kidney or liver disease requiring treatment, and absence of diseases involving peripheral nerves. Exclusion criteria included the withdrawal of consent, uncontrolled seizure disorder, injection site infection, and allergies to the study drugs. After explaining the study protocol to the patients, informed consent was taken. All patients who were eligible to enter the study were randomized into two groups by block randomization (freely available at: www.randomizer.org). Patient allocation was then concealed with the use of a sealed opaque envelope. Envelopes were opened by a pain fellowship in charge of injection before the intervention to clarify the type of injection. Patients then underwent the intervention. After the intervention, the outcomes were assessed by another physician. Patients and outcome assessors were blinded to the type of intervention.
3.1. Anesthesia Protocol
All patients fasted for at least eight hours prior to the surgery. After entering the operating room, patients were monitored for Non-invasive Blood Pressure (NIBP), electrocardiogram (ECG), heart rate (HR), and blood oxygen saturation (SPO2). Patients received midazolam (0.03 - 0.05 mg/kg), fentanyl (3 µg/kg), propofol (1 mg/kg), and then atracurium (0.5 mg/kg). After intubation, they received isoflurane (1 MAC) intraoperatively. Then, patients underwent End-Tidal CO2 (ET CO2) monitoring, which was maintained at 35 - 45 mmHg during surgery. The tidal volume was kept at 7 - 10 mL/kg. Remifentanil (0.1 µg/kg/min) was infused during surgery. At the end of the operation and after extubation, the patients were transferred to the recovery room.
3.2. Ultrasound-guided Intercostal Nerve Block
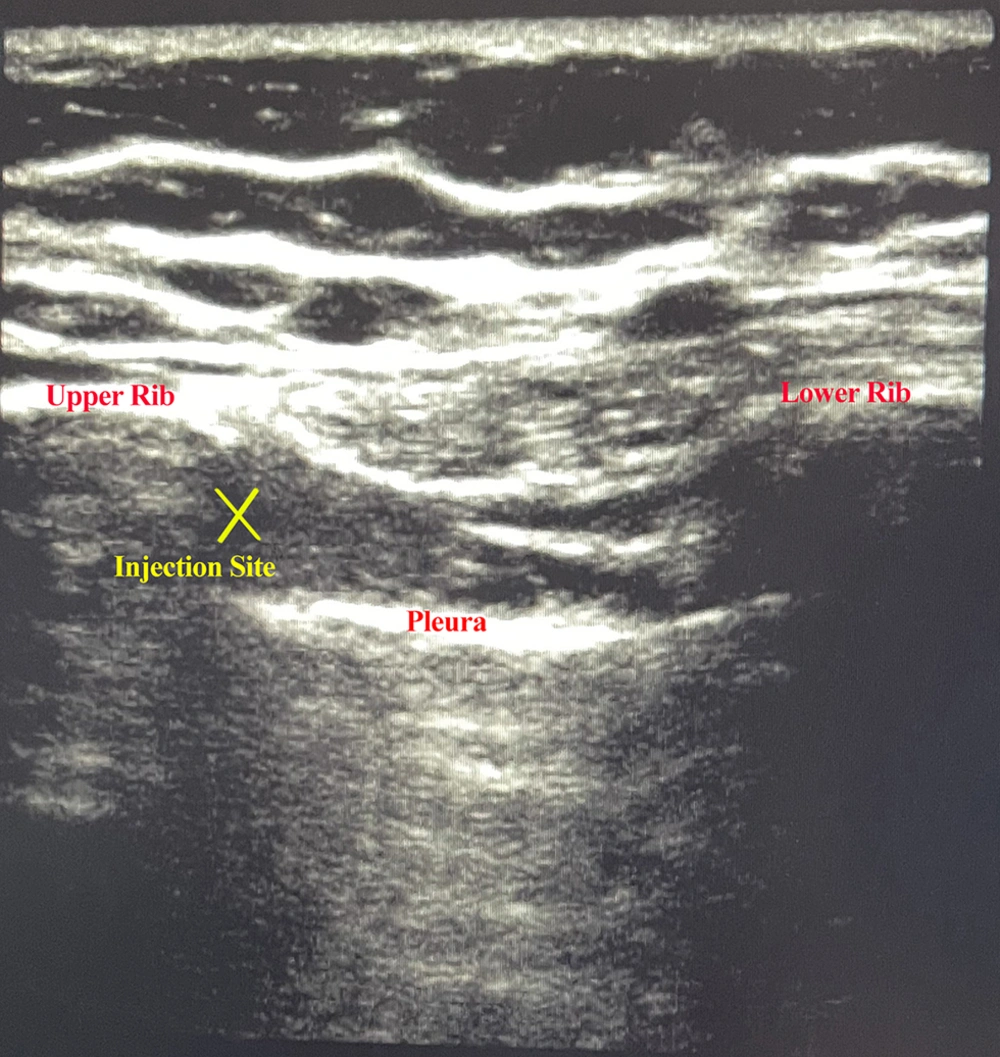
In recovery after hemodynamic stability, an ultrasound-guided intercostal block (M7, MindRay, China) was performed by a pain fellowship. Group R received ropivacaine (Molteni, Italy) 5 cc of 0.25% solution, and group RD received ropivacaine (Molteni, Italy) 5 cc of 0.25% solution plus 0.5 µg/kg dexmedetomidine (Medonex 200 µg/2 mL, Exir Pharmaceutical, Iran). Next, the prep and drape intercostal block was performed in a sterile manner in the supine position along the posterior axillary line. The linear (high frequency) transducer was placed in the longitudinal plane. After identifying the external intercostal muscle on the outside, internal intercostal muscle, innermost intercostal muscle, upper and lower rib, and pleura, a Quinke spinal needle gauge 22 (Dr. Japan, Japan) was inserted in the in-plane view until reaching the lower edge of the rib between internal and innermost intercostal muscles and it was aspirated. If no blood or air entered the syringe, the local anesthetic solution was injected. Figure 2 shows the ultrasonographic view of injection. This process was repeated in two intercostal spaces above and two intercostal spaces below the level of surgical incision. The pain fellowship responsible for performing the intercostal block was aware of the injected drug but did not participate in the data collection and analysis process. The patient was monitored after transfer to the ICU using non-invasive methods, and the study measures were recorded.
3.3. Measures
The data, including pain score, total opioid intake, length of ICU stay, and time to get out of bed, were recorded on a checklist for each patient. Prior to surgery, the patient's height and weight were measured and added to the patient checklist. The duration of surgery was recorded at the end of surgery and noted in the patient checklist. Study variables were measured six times (time 0 before, first hour, sixth hour, 12th hour, 24th hour, and 48th hour after the intervention). Patient pain was assessed using the Verbal Numerical Rating Scale (VNS). This scale is rated from 0 to 10, where 0 indicates no pain and 10 indicates the worst pain imaginable. When the VNS score was above 3, the patient was given pethidine (1 mg/kg) or opioid equivalent intravenously. The total opioid consumed by each patient was recorded.
3.4. Statistical Analysis
The sample size was calculated based on the study by Agamohammdi et al. (28), where the mean difference in pain scores between the bupivacaine group and the bupivacaine plus dexmedetomidine group was reported to be 2.0, and the highest standard deviations observed in the groups were 3.5 and 2.4, respectively. Hence, 37 patients per group were required to ensure an 80% power at α = 0.05. Statistical analysis was performed using SPSS (version 18.0, IBM) and Prism (version 8, Graphpad). The outputs of the analyses were expressed as mean ± standard deviation. The comparisons between and within groups (at various times) were performed using the one-way Analysis of Variance (ANOVA) and repeated measures ANOVA. The comparison between the number of patients was made using the chi-square test. The significance level in all tests was set at a p value of less than 0.05.
4. Results
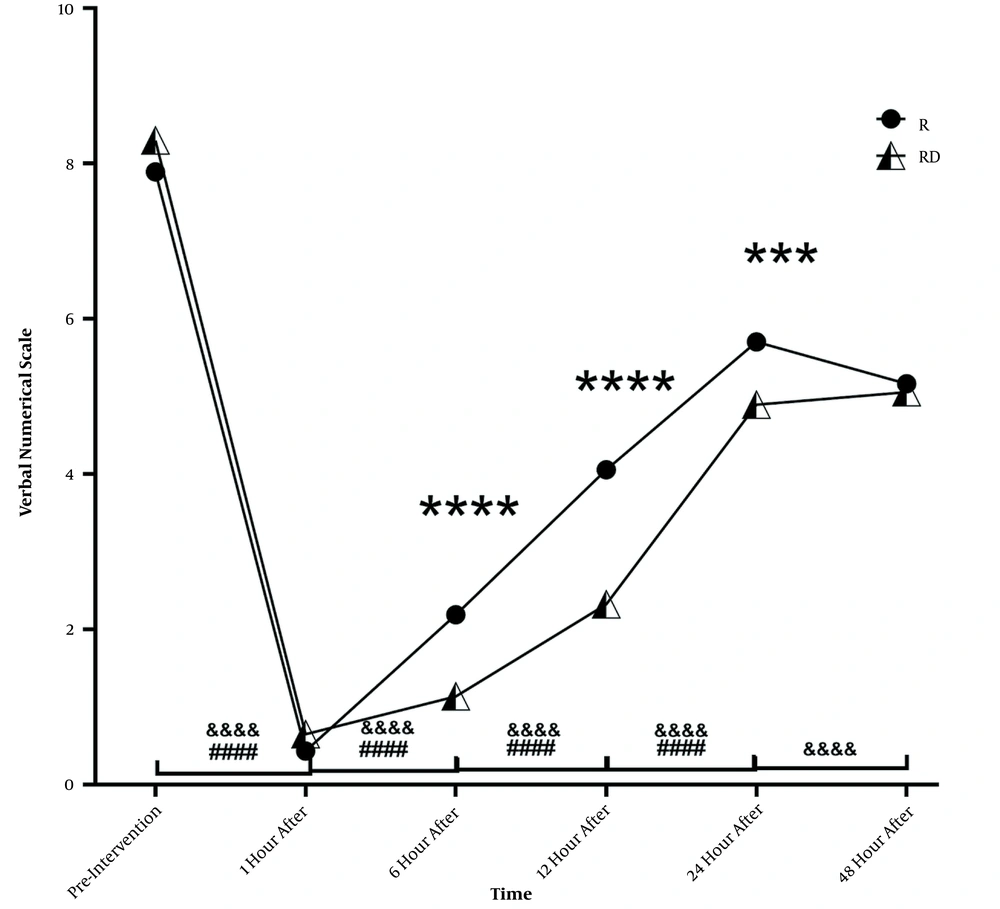
Of 74 patients enrolled in this study, 44 (59.46%) were males, and 30 (40.54%) were females. The mean age of the participants was 40.57 ± 12.20 (range 19 to 60) years. The patients’ demographic characteristics and operation time are presented in Table 1. There were no significant differences between the groups in age, height, weight, BMI, sex, and operation time (P value > 0.05). Table 2 and Figure 3 compare the mean VNS score between the two groups. At the beginning of the study and before the block, the RD group reported more pain than the R group, although this difference was not significant (P = 0.201). One hour after the intervention, there was a significant reduction in pain in both groups (a decrease of 7.459 units in the R group and 7.649 units in the RD group; P < 0.0001). At the sixth hour, the VNS score showed an increase of 1.757 units in the R group and 0.486 units in the RD group. At this time, the difference between the two groups was significant (P < 0.0001). At the 12th hour, the pain continued to increase (1.865 units in the R group and 1.189 units in the RD group). At this time, the difference between the two groups was significant (P < 0.0001). At the 24th hour, the R group continued to experience increased pain with the same intensity as before (1.676 units), but in the RD group, the amount of pain at this hour showed a sudden surge (2.568 units increase). At the 24th hour, the difference between the two groups was still significant (P < 0.001). Finally, there was no significant difference between the two groups in the final measurement at the 48th hour (P = 0.5393). Except for VNS at the 48th hour in the RD group (P = 1.000), the difference between all measurements was significant in both groups (P < 0.001).
| Measure | Assigned Group | P-Value | |
|---|---|---|---|
| R | RD | ||
| Age | 39.7 ± 12.0 | 41.3 ± 12.4 | 0.584 |
| Height (cm) | 172.6 ± 8.4 | 173.1 ± 9.3 | 0.815 |
| Weight (Kg) | 82.1 ± 13.8 | 85.2 ± 13.5 | 0.329 |
| BMI (Kg/m2) | 27.4 ± 3.2 | 28.3 ± 3.6 | 0.221 |
| Sex (M/F) | 24/13 | 20/17 | 0.344 |
| Operation time (hour) | 2.5 ± 0.96 | 2.7 ± 1.1 | 0.272 |
Abbreviations: R, ropivacaine; RD, ropivacaine plus dexmedetomidine.
aValues are expressed as mean ± SD.
| Time | VNS (Assigned Group) | P-Value | |
|---|---|---|---|
| R | RD | ||
| Pre-block | 7.8 ± 1.3 | 8.3 ± 1.3 | 0.2 |
| First hour | 0.4 ± 0.6 | 0.6 ± 0.7 | 0.164 |
| Sixth hour | 2.1 ± 0.7 | 1.1 ± 0.8 | < 0.0001 |
| 12th hour | 4.0 ± 0.9 | 2.3 ± 1.0 | < 0.0001 |
| 24th hour | 5.7 ± 0.9 | 4.8 ± 1.0 | 0.0008 |
| 48th hour | 5.1 ± 0.7 | 5.0 ± 0.7 | 0.539 |
Abbrevitions: VNS, Verbal Numeric Rating Scale; R, ropivacaine; RD, ropivacaine plus dexmedetomidine.
Pain comparison at measurement times between R and RD groups, ****, P < 0.0001; ***P < 0.001 between two groups; ####, P < 0.0001 in group RD compared to previous measurement; &&&&, P < 0.0001 in group R compared to previous measurement. Abbreviations: R, ropivacaine; RD, ropivacaine plus dexmedetomidine.
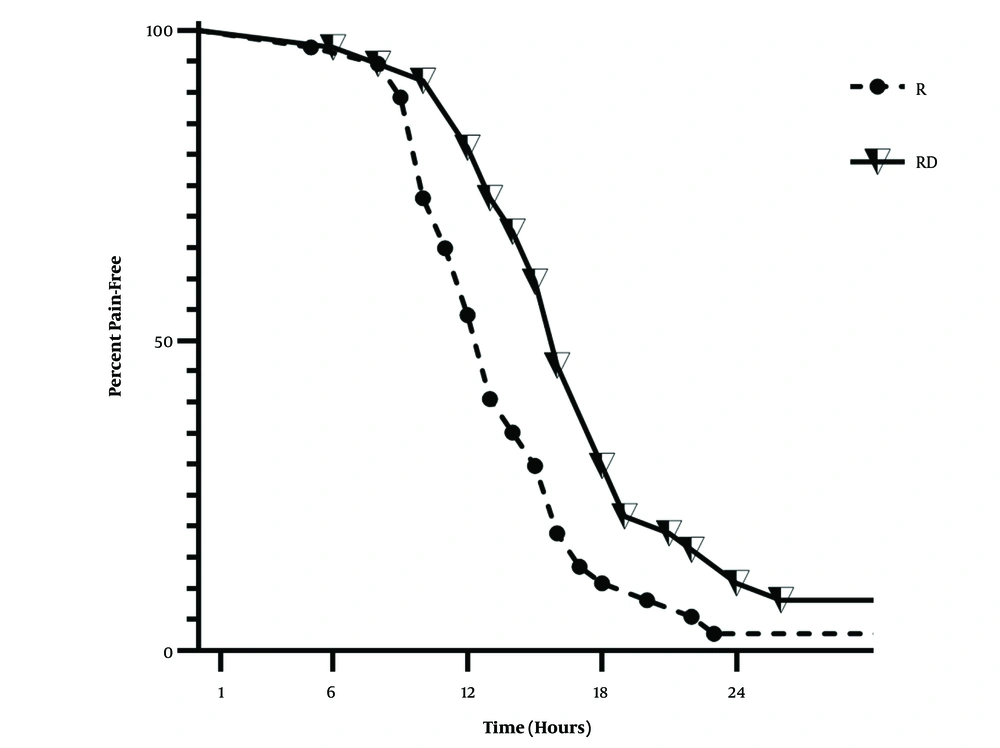
Figure 4 and Table 3 compare pain-free patients between the two study groups. The log-rank test showed that the two groups were significantly different (P = 0.0037, and χ2 = 8.433), and patients in the RD group were pain-free for a longer period. The median time to rescue analgesia was 13 hours in the R group and 16 hours in the RD group. Maximum pethidine consumption was significantly lower in the RD group than in the R group (190 mg vs. 235 mg, P value = 0.0003). The time to injection of rescue analgesics was significantly longer in the RD group, but there was no significant difference in the ICU stay and time to get out of bed (P value > 0.05). Table 4 shows the comparison between these values.
| Time | Pain-free Patients (Assigned Group) | P-Value | |
|---|---|---|---|
| R | RD | ||
| First hour | 37 | 37 | 1.0000 |
| Sixth hour | 37 | 37 | 1.0000 |
| 12th hour | 24 | 34 | 0.0050 |
| 24th hour | 2 | 6 | 0.136 |
| 48th hour | 1 | 3 | 0.3070 |
Abbreviations: R, ropivacaine; RD, ropivacaine plus dexmedetomidine.
| Measure | Assigned Group | P-Value | |
|---|---|---|---|
| R | RD | ||
| Time to rescue analgesic | 13.2 ± 3.8 | 16.0 ± 4.4 | 0.584 |
| N | 36 | 34 | |
| ICU stay (day) | 3.2 ± 2.6 | 3.2 ± 2.5 | 0.815 |
| N | 37 | 37 | |
| Time to get out of bed (day) | 4.2 ± 3.1 | 4.3 ± 3.3 | 0.329 |
| N | 37 | 37 | |
| Total opioid consumption (mg) | 180 ± 55.5 | 130.1 ± 57.9 | 0.221 |
| N | 37 | 37 | |
Abbreviations: R, ropivacaine; RD, ropivacaine plus dexmedetomidine.
aValues are expressed as mean ± SD unless otherwise indicated.
Two patients in the RD group developed bradycardia (50 < heart rate < 60), which was resolved without any treatment. No bradycardia was observed in the R group.
5. Discussion
Dexmedetomidine is an alpha-2-agonist whose effects have been extensively studied, which include the reduction of preoperative stress and inflammation, improvement of gastrointestinal function, reduction of opioid use, and prolongation of analgesia in patients after various surgeries (20, 29-34). The patients in our study had higher age and BMI than in many previous studies (31, 35, 36). The intercostal block with ropivacaine was very effective in both groups of patients and significantly reduced pain during the first hour after the intervention; this reduction was evident even up to 48 hours later. The addition of dexmedetomidine to ropivacaine prolonged the analgesic effect and reduced pain in this group compared to the ropivacaine group in the first 24 hours of the study. The dexmedetomidine group had less pain than the ropivacaine group at the sixth (P < 0.001), 12th (P < 0.001), and 24th hours (P < 0.01). This finding was similar to the study by Yao et al. (37), who compared two different routes of dexmedetomidine administration and showed that the perineural administration of dexmedetomidine at 0.5 μg/kg led to significantly lower pain at the 12th hour after lumpectomy. Also, Shen et al. (38) showed similar results in adenomyomectomy when comparing the low, medium, and high doses of dexmedetomidine. In this study, the VAS score was significantly lower in the moderate and high-dose groups than in the control and low-dose groups (P < 0.05), while no statistical difference was observed between the medium and high-dose groups (P > 0.05). Similar results were also reported by Abdallah et al. (39) for the addition of dexmedetomidine to levobupivacaine in the serratus anterior plane block, where the amount of pain reported in the dexmedetomidine group was significantly lower at six hours after the block up to 24 hours after. In a study by Talebi et al. (40), a combination of dexmedetomidine (0.5 μg/kg) and 20 cc of Marcaine 0.125 was used in the Transverse Abdominis Plane (TAP) block under ultrasound guidance, and the addition of dexmedetomidine resulted in the increased duration of the block, less pain during the first 24 hours, and less opioid use. There was no use of rescue analgesia in the first four hours, which was similar to our study. Margulis et al., who compared the addition of dexmedetomidine and dexamethasone as adjuvants to ropivacaine in ultrasonic-guided arthroscopic shoulder surgery, found that adding dexmedetomidine reduced opioid use in the first 48 hours, similar to our results (41). Omar Mostafa et al., who used 1 μg/kg dexmedetomidine as an adjuvant to bupivacaine in the paravertebral block to control postoperative pain in mastectomy, found that this addition increased the time to first rescue analgesia (42).
The addition of dexmedetomidine did not make a significant difference between the groups in terms of time to get out of bed and the length of ICU stay, but reduced the total amount of opioid consumption (P < 0.01) and increased the time to first rescue analgesia (P < 0.05). These findings confirm the report of Agamohammdi et al. (28), who compared the effect of bupivacaine and the combination of dexmedetomidine with bupivacaine in 64 patients with multiple rib fractures and reported a longer pain reduction effect in the dexmedetomidine group. On average, the dexmedetomidine group received 60 mg less opioid, and the first use of rescue analgesia happened about 2.5 hours later. Similarly, the study by Akhondzadeh et al. used 1 μg/kg dexmedetomidine as an adjuvant to lidocaine in the supraclavicular block. This addition increased the time to first rescue analgesia and decreased total opioid consumption compared to lidocaine alone (43). Many studies had already stated this effect of dexmedetomidine, as noted in a systematic review by Habibi et al. (44).
5.1. Conclusions
Dexmedetomidine is an effective and safe choice that can be used as an adjunct to ropivacaine in ICB. The combination of dexmedetomidine with ropivacaine for the intercostal nerve block can prolong the duration of analgesia after thoracotomy.