1. Background
Recently, among multiple surgical techniques for the treatment of cholelithiasis and cholecystitis, laparoscopic cholecystectomy (LC) is a method of choice (1, 2). Both neuraxial and general anesthesia are common anesthetic techniques in these patients. Various physiological changes may occur during anesthesia for laparoscopic surgery, which may lead to hemodynamic instability, increase in intra-abdominal pressure caused by pneumoperitoneum (CO2 insufflation), and patient position (3). Following the painful irritations that occur after the induction of anesthesia and during surgery, the patient’s stress responses are triggered. Hence, choosing a method that minimizes the hormonal fluctuations caused by stress responses will be desirable (4). Today, one of the serious concerns of researchers and physicians is to find effective medications to control the increase of inflammatory and stress responses. Epinephrine is a neurotransmitter of the endogenous catecholamines group, which causes an increase in heart rate, vasoconstriction, and dilatation of airways (5).
Propofol generates the most pronounced decrease in systemic blood pressure compared with other anesthetics, which, in addition to rapid anesthetic effects and lower side effects, has a faster anesthesia return than other intravenous anesthetics (6-8); it is also known as the best anesthetic drug for continuous infusion due to its rapid metabolism and the lack of cumulative effects. After the induction of anesthesia, propofol may result in a decrease in blood pressure and bradycardia due to less inhibition of the parasympathetic nervous system compared to sympathetic one.
The use of preoperative α2 receptor agonists improves hemodynamic stability due to its numerous beneficial effects, including analgesic effects, inhibition of sympathetic outputs, anti-anxiety properties, and reduction of norepinephrine levels (9). They protect myocardial muscle because of positive effects on myocardial oxygen supply and cardiac oxygen demand (10-13). Stress response involves various hormones, such as release of epinephrine, cortisol, some cytokines like interleukin, TNF, and growth factors, and complement system activation. This stress response to surgery can be reduced by activating an alpha-dual adrenergic receptor (14, 15). Dexmedetomidine is a highly selective α2 receptor agonist with 1600-fold affinity to α1 receptor (16). The use of dexmedetomidine before anesthesia has a positive effect on hemodynamic stability, which has been associated with reduced postoperative mortality and reduction of unpleasant postoperative complications (17-20). The results of laboratory and clinical studies showed that dexmedetomidine reduces inflammatory responses (21, 22), and animal studies also showed inhibition of pro-inflammatory cytokines (23). In addition, the results of in vitro studies on whole human blood samples reported suppression of lipopolysaccharides, which produce pro-inflammatory mediators including TNF-α, interleukin-6, and IL-8 (24, 25). Besides vasodilatory effects, alpha-2 agonists have sympathetic suppressive, sedative, and hypnotic effects (26). Alpha-2 agonists, such as clonidine, have a blood pressure lowering effect, and therefore reduce surgical bleeding (16, 27); in addition, dexmedetomidine facilitates analgesia and anesthesia in humans and reduces the severity of postoperative pain and nausea (28-30). This drug is approved for use for up to 24 hours at a maximum dose of 0.7 micrograms per kilogram of body weight per hour (31). Several studies showed that dexmedetomidine is relatively safe even after long-term administration in high doses, with few side effects (32). Intravenous dexmedetomidine reduces the need to use high dose of propofol administration to achieve and maintain desirable bispectral index score (BIS) with low side effect (33).
2. Objectives
Considering the beneficial effects of propofol and dexmedetomidine, we aimed to study their effects on the identical BIS, hemodynamic parameters, and inflammatory and stress factors in patients undergoing LC surgery.
3. Methods
3.1. Study Design
After approving the research in the Ethics Committee of Jundishapur University of Medical Sciences of Ahvaz (code: IR.AJUMS.REC.1398.778) and obtaining an informed consent from the patients, the study was conducted as a double-blind randomized clinical trial (patient and bio-statistician were blinded). According to the criteria by the American Society of Anesthesiology (ASA) class I-II, we included 75 patients (age range: 20 - 60 years) undergoing elective LC surgery. Patients were divided into two equal groups: propofol (n = 34) and dexmedetomidine (n = 34).
Common standard monitoring measurements, including electrocardiogram (ECG), blood pressure, pulse-oximetry, end-tidal CO2 capnometry (Model M3B Edan), and BIS (Cerebral State Monitor, Model CSM 2Danmeter A/S) were performed upon the entry of patients to the operating room. Demographic information (age, sex, height, weight) and changes in hemodynamic parameters (mean atrial pressure [MAP], heart rate) and end-tidal CO2 were recorded. The patients were oxygenated at a rate of 5 liters per minute with 100% oxygen for 3 min. All patients received a combination of midazolam (0.05 mg/kg; Chemidarou Iran Co.), fentanyl (2 μg/kg; Aburaihan Iran Co.), sodium thiopental (4 mg/kg; Trittau, Germany), and atracurium (0.5 mg/kg; Caspian Tamin) for induction and morphine (0.1 mg/kg; Darupakhsh Iran Co.) was administered for analgesia after induction. The maintenance of anesthesia in propofol group was infusion of propofol at a dose of 75 μg/kg/min (Dongkook pharm. co. ltd, Korea), and in the dexmedetomidine group was infusion of dexmedetomidine at a dose of 0.5 μg/kg/hour (Exir Co. ltd Iran). Atracurium (0.1 mg/kg) was used alternately every 20 min as a relaxant (34). The depth of anesthesia in both groups was maintained between 45 and 50 BIS through the infusion of propofol and dexmedetomidine. Both groups received remifentanil (0.7 µg/kg/min; intravenous [IV] after intubation; Fresenius SE & Co KgaA, India) until the end of surgery.
This study was carried out at Golestan Hospital of Ahvaz Jundishapur University of Medical Sciences, Iran, from January 2020 to February 2021.
3.2. Study Patients
In this study, a total of 75 consecutive patients scheduled for elective LC were enrolled (Figure 1). Inclusion criteria were: not having urgent/emergency surgery, obesity grade II or III (BMI > 35), chronic liver disease, diabetes, renal failure, endocrine problems (pheochromocytoma), rheumatic disease, cardiovascular disease or malignancy, and egg allergy; patients receiving drugs with known effects on sympathetic response or hormonal secretion such as beta blockers, epinephrine, and insulin; patients consuming benzodiazepines, dextrose serum, and dexmedetomidine; and pregnant or lactating mothers. Exclusion criteria were: unwillingness to participate in the study and changing the method of surgery to classical open cholecystectomy.
3.3. Study Variables
Demographic data (age, sex, and BMI) and duration of anesthesia and surgery were recorded. Patients’ hemodynamic parameters, including heart rate (HR) and MAP, were recorded during the following stages of the study: pre-induction, induction time, and intra-operatively every 5 min till the end of anesthesia and 10 min after PACU entry.
Plasma level of epinephrine and blood glucose (BS) had been measured immediately after induction and at the end of surgery (before reversal of neuromuscular block).
3.4. Laboratory Measurements
Before and after each surgery, blood samples were obtained, and plasma was prepared. Epinephrine level was measured by human epinephrine ELISA kit (Taoyuan, Taiwan; Catalog Number: KA1877), with 18 to 6667 pg/mL normal value range. Glucose was measured by a biochemical colorimetric method (Karaj, Iran, Parsazmon; Catalog Number: G45215)
3.5. Statistical Analysis
Categorical variables were reported as percentages, and continuous variables were reported as the mean ± standard deviation or median (interquartile range). The assumption of normality was evaluated using the Shapiro–Wilk or Kolmogorov–Smirnov test. Epinephrine and glucose levels were compared using the analysis of variance (ANOVA) and Tukey’s post-hoc tests. HR and MAP were analyzed by repeated measures ANOVA (RM-ANOVA) test. Data were analyzed using GraphPad prism® software version 8.3.0.
4. Results
A total of 68 patients (n = 34 in each group) were studied. The median age was 54 years in the propofol group (median + IQR, 54, 49 - 58) and was 56 years in the dexmedetomidine group (median + IQR, 56, 48 - 59). In propofol group, 58% of the patients (n = 20) and in dexmedetomidine group, 69.58% of the patients (n = 24) were females. The demographic data are listed in Table 1. As can be seen, weight was higher in the dexmedetomidine group (P = 0.019), but there was no significant difference in other variables between the two groups.
| Variables | Propofol (n = 34) | Dexmedetomidine (n = 34) | P-Value |
|---|---|---|---|
| Gender | 0.621 | ||
| Female | 20 (58) | 24 (69.57) | |
| Male | 14 (42) | 10 (30.43) | |
| Age (y) IQR | 54 (47 - 57) | 56 (46 - 60) | 0.360 |
| Height (cm) | 165.8 ± 5.15 | 166.94 ± 5.51 | 0.857 |
| Weight (kg) | 67.9 ± 6.06 | 72.88 ± 7.50 | 0.019* |
aValues are expressed as No. (%) or mean ± SD.
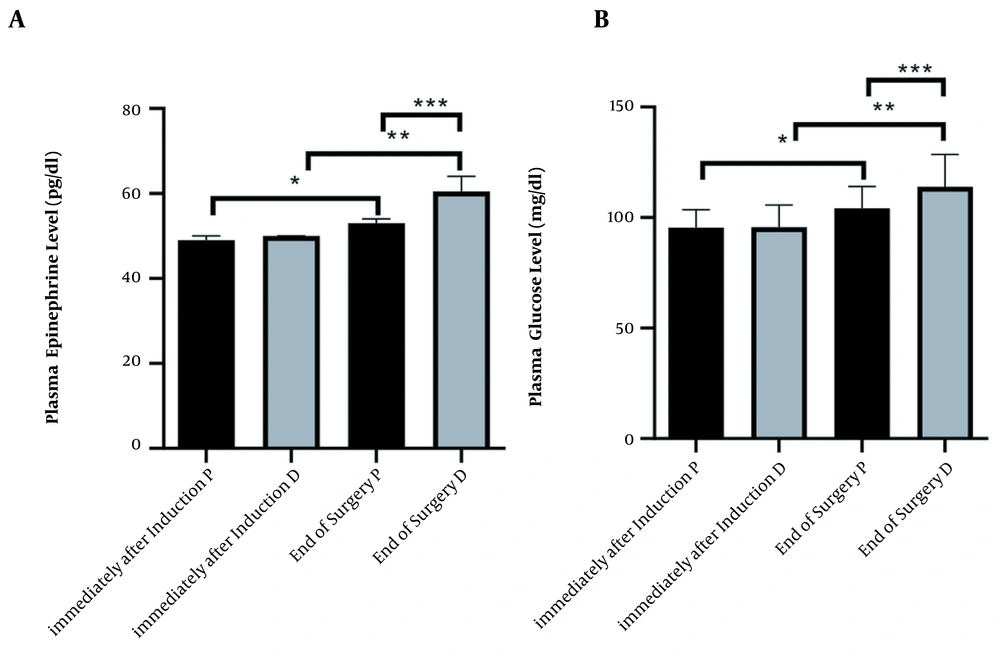
Epinephrine level in the different times of immediately after induction and end of surgery, in propofol and dexmedetomidine groups, was (mean ± SD, 95.38 ± 8.14 ,104.1 ± 9.87, 95.74 ± 9.94, 114.0 ± 14.58, respectively). ANOVA test showed a significant difference between all times and agents (P < 0.0001) (Figure 2A).
Changes immediately after induction and end of surgery in two groups under maintenance of anesthetic propofol (P, n = 34) and dexmedetomidine (D, n = 34) administration. (A) Plasma level Kruskal–Wallis test analysis (P < 0.0001), (*, ** and ***P < 0.0001). (B) Plasma level of glucose ANOVA analysis (P < 0.0001), (*, ** and *** P < 0.0001)
Glucose level in the different times immediately after induction and end of the surgery, in propofol and dexmedetomidine groups was (mean ± SD, 49.06 ± 1.04, 53.50 ± 3.73, 49.71 ± 1.21, 61.38 ± 3.70, respectively). ANOVA test showed a significant difference between all times and agents (P < 0.0001) (Figure 2B).
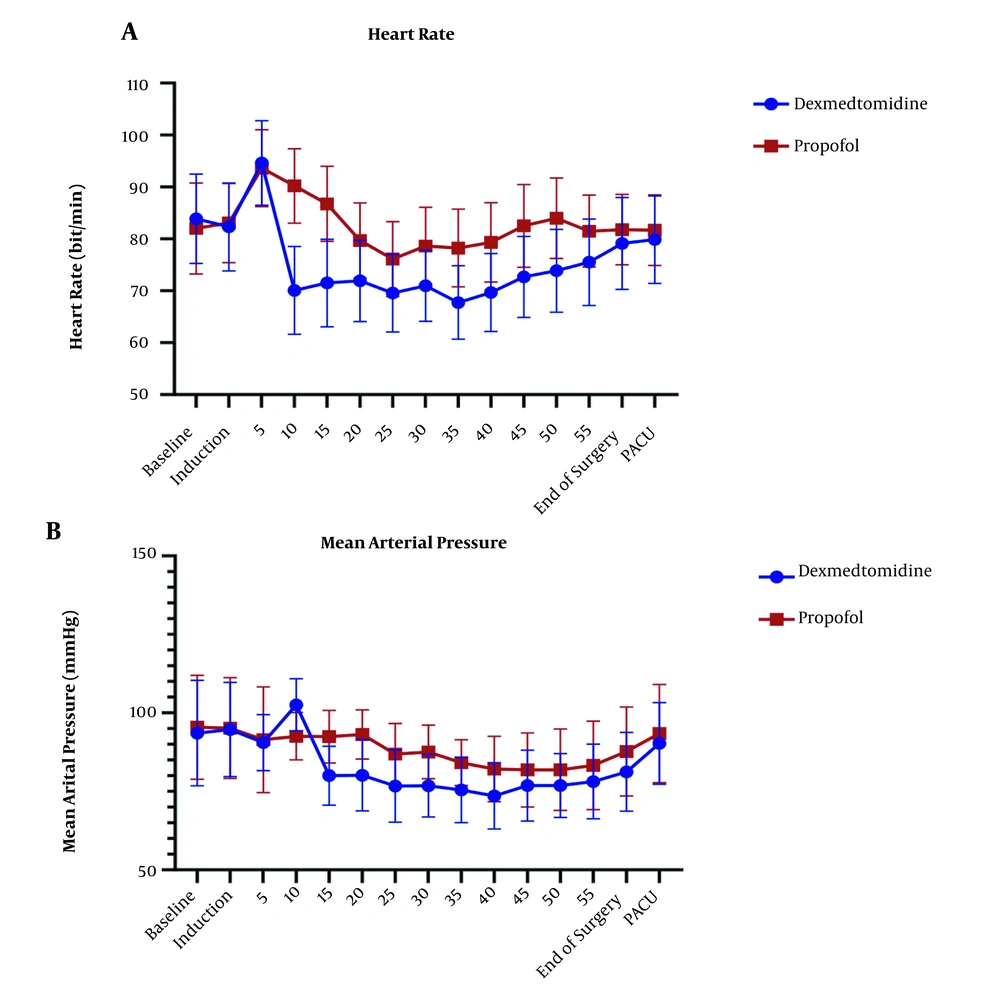
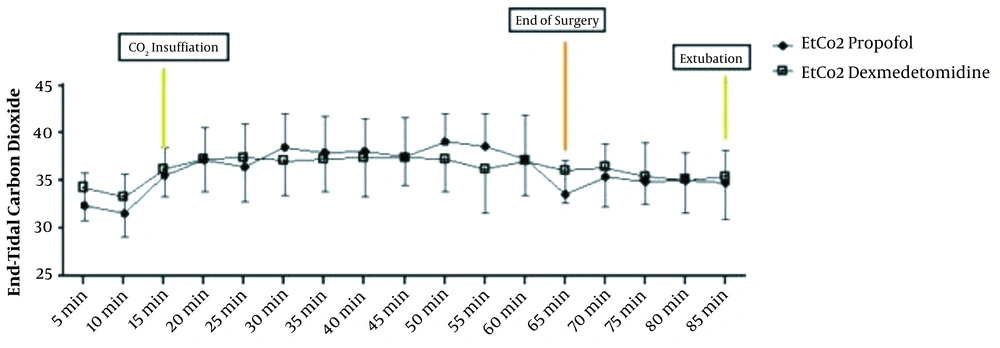
The repeated measure ANOVA test for analysis effect of time and agent showed significant difference in HR and MAP (P < 0.001) and no significant difference in EtCO2 (P = 0.636) (Figures 3 and 4).
(A) Changes in heart rate (HR) in the P group (n = 34,) and the D group (n = 34). Preoperative (-20), operative, and postoperative (PACU) measurements. P < 0.001 (Repeated measure ANOVA). (B) Changes in mean arterial pressure (MAP) in the P group (n = 34) and the D group (n = 34). Preoperative (-20), operative, and postoperative (PACU) measurements. P < 0.001 (Repeated measure ANOVA)
5. Discussion
5.1. Epinephrine and Glucose
Although surgical techniques have been developed, general stress response is an inseparable part of surgery (35). This stress is induced by surgical incision and is a complex of reactions that happen in the site of incision, such as increases in cAMP, epinephrine, and other catecholamines (36). Reduction of physiologic stress response is an anesthesiology goal for maintaining a patient under stable hemodynamic level during the surgery. In molecular view, this response induces biochemical changes, including epinephrine, norepinephrine, cortisol, ACTH, IL1, and IL6 rise, which are detectable in the blood as the central compartment of the body (34). Epinephrine is a rational index in surgical stress. In comparison to conventional cholecystectomy, LC has a low stress response (Table 2) (37, 38). According to the study by Glaser et al., in different types of cholecystectomy surgery (classic versus LC) catecholamine changes has the same trend (39). In this study, epinephrine was selected as a rational index, and glucose was measured as an indirect marker of changes in catecholamines. Although epinephrine levels increased in both groups, the propofol group had significantly low-stress profile than the dexmedetomidine group. The trend of glucose level changes was similar to the epinephrine trend. Dexmedetomidine, as the only anesthetic agent, did not have an appropriate protective effect on stress hormones (40). This may be due to a different pharmacodynamic mechanism compared to propofol. Dexmedetomidine induces deep sleep via noradrenergic locus ceruleus neuron hyperpolarization compared to agonistic action on GABAa receptors pathway by propofol (41, 42).
| Type of Surgery | Researcher | Year of Publication | Variables Measured | Anesthesia Methods | Results | References |
|---|---|---|---|---|---|---|
| Laparoscopic surgery | ||||||
| Cholecystectomy | Glaser et al. | 1995 | The level N, E, ACTH, Cortisol LC versus CC | Mixed | LC ↓ in stress response versus CC | (37) |
| Non- cholecystectomy | Marana et al. | 2010 | N, E, ACTH, Cortisol, GH, PRL, TSH, FT3, FT4 | Induction: STP; Maintenance: Propofol vs. sevoflurane | In propofol group level E ↓, Sevoflurane group: E ↑ | (43) |
| Azemati et al. | 2013 | CRP, Glucose, cortisol, HR. MAP | Maintenance: Propofol vs. isoflurane | In propofol group level E ↓, Isoflurane group: E ↑ | (44) | |
| Non- laparoscopic surgery | ||||||
| Not define type of surgery | Adams et al. | 1994 | N, E, ACTH, Cortisol, ADH, HR and arterial pressure was measured | Induction: STP and Propofol; Maintenance: Propofol vs isoflurane | Level EPI in propofol group significantly was lower | (45) |
| Ihn et al. | 2009 | N, E, ACTH, Cortisol, Glucose and Il-6 | Induction: STP; Maintenance: Propofol vs. sevoflurane | Level E and Glucose ↑ in sevoflurane; Level E and Glucose in propofol ↑ and is lower than sevofluarne | (46) | |
| Bulow et al. | 2007 | Cortisol, Blood sugar | TIVA by propofol; Comparison: DEX vs remifentany | Level BS and cortisol was higher in DEX group | (47) |
5.2. Hemodynamic Parameters
The HR level had a statistically significant difference in all stages of measurement after induction in the two groups. After induction of anesthesia, the groups had the opposite trend, dexmedetomidine has declining trend of HR changes, but propofol has increased this trend. Trend confusion in 25-35 minute after induction could explain by surgery manipulation. In a non-major type of surgery study by chattopadhyay et al. (41), dexmedetomidine group had lower HR in comparison to propofol, which was consistent with the results of the present study. In a study by Azemati et al. (44), after induction, the mean HR significantly decreased in both propofol groups compared to baseline, and remained below baseline until the end of the surgery, which had the same results as our study.
The mean of MAP has shown a statistically significant difference (P > 0.05) in both groups and had lower amount in dexmedetomidine group, that back to mechanism of alpha 2 agonist characteristic of dexmedetomidine. Many studies showed dexmedetomidine alone or as adjuvant could control blood pressure (41, 47-49). Tilvawala et al. (50) compared the effect of propofol and sevoflurane on hemodynamic changes in laparoscopic surgeries; they reported that there was no significant difference between the two groups in hemodynamic changes. In a study by Azameti et al., arterial blood pressure was significantly reduced in both propofol and isoflurane groups, which is consistent with the results of this study, in which only the propofol receiving group had a significantly lower MAP at the end of surgery (44). In the propofol group, the mean of MAP at the end of surgery was significantly lower than baseline, and in the isoflurane group, this factor was not significantly different from baseline (51). After the end of the surgery, the mean blood glucose in the patients receiving propofol was significantly lower than that in the dexmedetomidine group, and dexmedetomidine group had higher amount in glucose level at the end of surgery point in comparison itself in immediately after induction points.
In a study by Azemati et al., glucose was significantly increased in both groups receiving propofol and isoflurane, but one hour after cutting but one hour after incision of the surgeon and one hour after the end of the surgery, the amount of glucose decreased significantly in propofol group (44).
At the end of the surgery, the mean epinephrine plasma levels in patients receiving propofol were significantly lower, and the increase in epinephrine levels in the dexmedetomidine group was significantly higher than that in the propofol group.
5.3. Conclusions
Although epinephrine level was low in propofol group, there was a significant difference in hemodynamic parameters that suggest future studding on the combination of dexmedetomidine propofol. Consistent hemodynamic and lack of significant difference in important factors could refer to monitoring depth anesthesia by BIS.