1. Background
The gut microbiota (GM) has a variety of influences on the health status of the host human (1). Also, it can be hypothesized that the GM may influence pain processing and perception. Several human and animal studies supporting this hypothesis have been conducted (2-5). Although these studies have reported the relationship between GM composition and disease characteristics, that was different from that of healthy controls, there were no reports about the relationship between pain perception and GM composition. We previously investigated this issue in healthy young males and reported that pain perception may be related to GM composition (5). However, no gender-based differences in the composition of GM has been reported (6).
Psychological factors are also involved in pain processing and stress. Mood and pain are interrelated, and it has been reported that patients with fibromyalgia have a higher prevalence of mild to moderate depression and anxiety (7). Moreover, psychological conditions such as anxiety and depression are associated with GM composition (8).
In our previous study conducted on males, pain perception showed a relationship with GM composition, but not with psychological factors (5). However, a higher number of females suffering from depression is both a universal and substantial trend (9).
2. Objectives
This study aimed to investigate the relationship between GM composition and pain perception in females by adding psychological factors as a secondary outcome.
3. Methods
3.1. Study Design
This prospective cohort study was conducted on Japanese females, and it was approved by the Nagoya Gakuin University Board of Ethics (No. 2016-27).
3.2. Subjects
The subjects of this study were 42 healthy females. The inclusion criteria were being at least 20 years old and having no ongoing pain or diseases. The exclusion criteria were people diagnosed with and treated for chronic diseases, those with active serious psychiatric disorders, those receiving drugs (e.g., analgesics or antibiotics), menstruating, pregnant, or lactating. Two subjects were excluded from the study due to menstruation. All participants signed an informed consent form.
3.3. Measures
Perception assessments comprised mechanical pressure pain threshold (PPT), current perception threshold (CPT), temporal summation of pain (TSP), and conditioned pain modulation (CPM). Psychological state was also evaluated using a questionnaire. In addition, GM composition was analyzed using 16S rRNA analysis. All subjects took part in the research twice a week. PPT, TSP, and psychological state were measured at the first visit, CPT, and CPM at the second visit, and stool sample was collected within three days of the first visit. All measures were performed within one week.
3.3.1. PPT
We used a pressure algometer (Digital Force Gage, AIKOH, Japan) to collect PPT. The site tested for PPT was the right dorsal forearm. The participants were asked to respond to the first onset of pain from the pressure applied, and the corresponding algometer force (newton) was recorded.
3.3.2. CPT
CPT was measured using a Neurometer® (Neurotron, Inc., USA). This device can produce frequencies of 2000, 250, and 5 Hz to selectively stimulate Aβ, Aδ, and C fibers, respectively. Measurements were recorded in the palmar of the pollicis at each frequency.
3.3.3. TSP
TSP was assessed by applying a mechanical pressure stimulus of 0.5 Hz to the dorsal forearm of the dominant arm for ten consecutive times. The intensity of the pressure stimulation was 1.25 times that of PPT. The participants rated the intensity of pain felt for each of the ten pressure stimuli on the visual analog scale (VAS), and the total change in the VAS was used as the TSP value.
3.3.4. CPM
CPM is an indicator of endogenous pain modulatory function. The conditioned stimulus was a 10±1°C cold water stimulus to the left hand, and the test stimulus was a PPT. CPM value was the rate of change in PPT before and 30 seconds after the start of the conditioned stimulus.
3.3.5. Psychological State
The Pain Catastrophizing Scale (PCS) was used to assess pain catastrophizing. Anxiety was evaluated using the State-Trait Anxiety Inventory Questionnaire (STAI; state anxiety: STAI-S, and trait anxiety: STAI-T).
3.3.6. GM Composition
GM analysis was performed using Mykinso Pro, a gut microbiome testing service (Cykinso, Inc., Japan). Excrement samples were compiled by participants using brush-type compiling kits containing guanidine thiocyanate solution (Techno Suruga Laboratory, Shizuoka, Japan), transported at normal temperature, and kept at 4°C until abstraction. Compiling and preservation of excrement samples were performed according to the manufacturer’s instructions. DNA abstraction from excrement samples was conducted using an automated DNA abstraction device (GENE PREP STAR PI-480, Kurabo Industries Ltd, Osaka Japan). The V1-V2 region of the 16S rRNA gene was amplified using forward primer and reverse prime. To sequence 16S amplicons by the Illumina MiSeq platform, dual index adapters were attached using the Nextera XT Index kit. The sequence library preparations were carried out according to 16S library preparation protocol by Illumina (Illumina, USA). Libraries were sequenced using the MiSeq Reagent Kit v2 (500 Cycles), 250 bp paired-end.
The paired-end reads of the partial 16S rRNA gene sequences were clustered by 99% nucleotide identity, and then assigned taxonomic information using Greengenes through QIIME 2 pipeline (version 2019.4)
Since a previous study on males found a relationship between pain perception and the rate of major phylum (Bacteroidetes, Firmicutes, Actinobacteria, and Proteobacteria) carriage (5), we focused on the same four major phylum in the present study. In addition, we added the analysis of alpha diversity (Shannon index), which has been reported to be associated with various chronic pain diseases (10, 11).
3.4. Statistical Analyses
First, we used GPower software to determine the sample size. The effect size was set at 0.50 based on the correlation coefficient between PPT and Bacteroidetes levels in our previous study (5). To show a significance level of 0.05 (α = 0.05) and a power of 95% (β = 0.05), a minimum of 42 subjects would be required.
All data were analyzed using SPSS version 26 (IBM, USA). The Shapiro-Wilk test was used to investigate whether the data were normally distributed. The correlation between each outcome was analyzed using Spearman’s rank correlation coefficient. Subsequently, a stepwise multiple regression analysis was performed using the Z-scores calculated from each measurement. We used the GM composition and psychological state as the explanatory variables due to their correlation with pain perception. In addition, we used pain perception as the objective variable in our multiple regression analysis. A P-value < 0.05 was considered statistically significant.
4. Results and Discussion
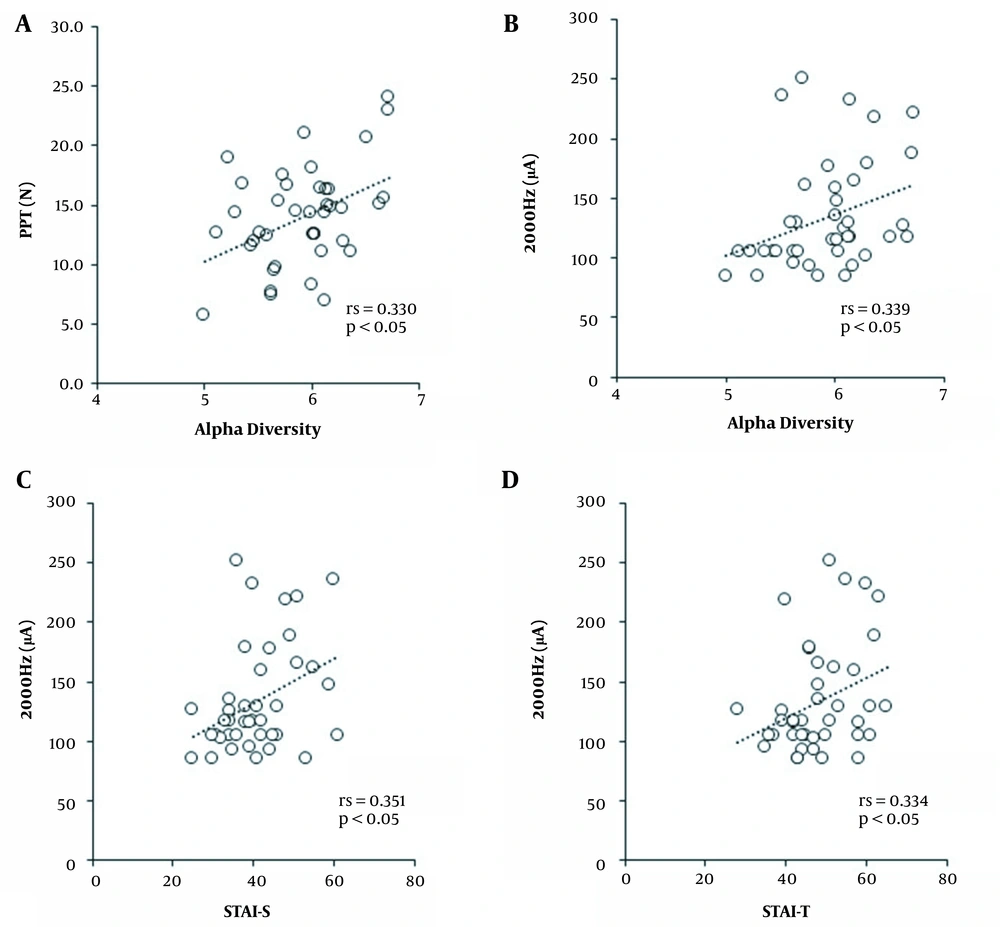
PPT (rs = 0.330, P = 0.037) and CPT of 2000 Hz (rs = 0.339, P = 0.011) positively correlated with alpha diversity (Figure 1A and B). The rate of major phylum carriage did not correlate with all pain perception indicators. Regarding the correlation between pain perception and psychological state, CPT of 2000 Hz positively correlated with STAI-S (rs = 0.351, P = 0.026) and STAI-T (rs = 0.334, P = 0.035) (Figure 1C and D).
A multiple linear regression was performed to predict pain perception assessment of the potential confounders (GM composition and psychological state) using the alpha diversity. STAI-S and STAI-T were found to correlate with pain perception as explanatory variables. The predictor of PPT was alpha diversity (β = 0.424, 95% CI: 0.127 - 0.721), the predictors of CPT of 2000 Hz were alpha diversity (β = 0.321, 95% CI: 0.030 - 0.611) and STAI-S (β = 0.359, 95% CI: 0.068 - 0.650), the predictor of CPT of 250 Hz was STAI-S (β = 0.320, 95% CI: 0.009 - 0.631), and that of TSP was alpha diversity (β = -0.317, 95% CI: -0.629 - -0.006) (Table 1).
| Dependent Variables | Explanatory Variables | Adjusted R2 | β | P-Value | 95% CI for β | |
|---|---|---|---|---|---|---|
| Lower Limit | Upper Limit | |||||
| PPT | Alpha diversity | 0.158 | 0.424 | 0.006 | 0.127 | 0.721 |
| CPT 2,000Hz | Alpha diversity | 0.197 | 0.321 | 0.032 | 0.030 | 0.611 |
| STAI-S | 0.359 | 0.017 | 0.068 | 0.650 | ||
| CPT 250 Hz | STAI-S | 0.079 | 0.320 | 0.044 | 0.009 | 0.631 |
| CPT 5 Hz | - | |||||
| TSP | Alpha diversity | 0.077 | -0.317 | 0.046 | -0.629 | -0.006 |
| CPM | - | |||||
Abbreviations: β, unstandardized coefficients; PPT, pressure pain threshold; CPT, current perception threshold; TSP, temporal summation of pain; PM, conditioned pain modulation; PCS, pain catastrophizing scale; STAI-S, state trait anxiety inventory; state anxiety.
The relationships between pain perception and GM composition in females showed that lower alpha diversity may be associated with lower PPT and CPT of 2000 Hz, indicating a higher sensitivity to mechanical pain and Aβ fibers. It is very interesting to note that Bacteroidetes and Firmicutes, which were found to be related to pain perception in a previous study on males (5), were not related to that in females. However, the previous study on males analyzed GM composition using QIIME1, which could not be directly compared to the present study because different analysis methods were used, and the alpha diversity was not measured.
High GM diversity is associated with good health (12), and Shannon diversity is one of the most common measures of GM diversity. Researchers have reported reduced alpha diversity in chronic widespread musculoskeletal pain (WAD) patients and in male patients with chronic pelvic pain syndrome compared to healthy subjects (10, 11). Fecal microbiota transplantation in patients with irritable bowel syndrome was reported to reduce abdominal pain and increase alpha diversity in these patients (13). However, these studies did not show a relationship between pain itself and GM diversity. This study may be the first to show that there is a possible relationship between pain sensitivity and the diversity of gut bacteria in young females.
The results of multiple regression analysis showed that high alpha diversity may be a predictor of reduced mechanical pain sensitivity. Also, low alpha diversity may be a predictor of high TSP, and psychological factors were not involved in these associations. TSP is an indicator of central sensitization, and increased TSP was associated with the development and maintenance of painful conditions in several clinical situations (14, 15). Therefore, the results of this study were significant because a decrease in alpha diversity might have an impact on the occurrence and persistence of pain. On the contrary, reduced alpha diversity was associated with lower pain sensitivity, as chemotherapy-induced mechanical hyperalgesia was reduced in germ-free mice and the mice pretreated with antibiotics (16). Since some animal studies have reported contradictory results, the relationship between alpha diversity and pain perception needs to be carefully interpreted.
Psychological factors that were not found in males were found to be involved in females. Moreover, the high alpha diversity and state anxiety were shown to be the predictors of low Aβ fiber sensitivity. In addition, high state anxiety was shown to be a predicator of low Aδ fiber sensitivity, indicating that psychological factors are associated with pain sensitivity in females. However, there was no synergistic influence of GM and psychological state on pain sensitivity.
Previous studies have reported that as anxiety increases, the intensity of pain increases (17), which seems to contradict the results of the present study. However, most of these reports have been conducted on patients with chronic pain, and there are very few reports showing the relationship between pain sensitivity and anxiety in healthy subjects.
It has been reported that anxiety disorders were associated with a reduction in microbial diversity and that improvement in anxiety was observed after taking probiotics (18). Also, gender differences in GM composition may be related to gender differences in childhood temperament, and anxiety states associated with female-specific hormonal changes may involve changes in the immune system due to changes in GM composition (18). Therefore, to clarify the relationship between GM composition, anxiety, and pain perception, as well as its gender differences, it is necessary to investigate other factors related to gender differences, such as hormones.
There were several limitations in this study. Firstly, we measured only the major phylum and alpha diversity, thus requiring a detailed evaluation of the GM composition. Secondly, as this study was conducted only on healthy young adults, it is necessary to include other groups of adults in future studies. Thirdly, it is necessary to investigate not only the relationship between GM composition and pain perception, but also the relationship with spontaneous pain in patients.
In conclusion, the present study showed an association between pain perception and GM composition in healthy young females. Our results suggested that lower alpha diversity may be associated with a higher pain perception and a higher TSP. The relationship between alpha diversity and pain perception needs to be further investigated, as there are some points that contradict previous reports.