1. Background
The SARS-CoV-2 pandemic is one of the greatest challenges facing healthcare providers, particularly in the critical care setting. Severe coronavirus disease 2019 (COVID-19) is characterized by hypoxia, respiratory distress, and failure (1). Keeping a safe airway in order to maintain ventilation and correct hypoxia is mandatory. The intubation rate was reported at 5 to 88%, and the extubation rate was 1.4 to 44.5% (2). Kangelaris et al. defined early intubation as intubation within 24 hours of admission and late intubation as intubation after 24 hours (3).
Some studies investigated the timing of intubation of COVID-19 patients. In critically ill patients, it was reported that early intubation had no survival benefits (4). Late-intubated patients had less lung compliance and ventilatory ratios than other patients. It can be related to self-induced lung injury or the progression of the disease (5). The meta-analysis results showed that intubation timing might not affect the mortality or morbidity of COVID-19 critically ill patients (6). However, it is hypothesized that early intubation may presumably decrease viral aerosols and droplets (7) and reduce the contamination. Besides, late intubation is likely to increase the risk of contamination during laryngoscopy and its supposed aerosol-generating consequences (8).
In acute respiratory distress syndrome (ARDS), the timing of intubation may be associated with therapeutic outcomes. In non-COVID ARDS, late-intubated patients had significantly greater fatality rates than those who were intubated early in the illness phase (3). Throughout the situation of a respiratory failure, early intubation in a controlled setting is also recommended (9). Gattinoni et al. recommended preventing patient self-induced lung injury (P-SILI) in support of the early intubation strategy (10). However, Tobin et al. criticized this idea and recommended avoiding the liberal use of early intubation (11). However, the advantages and disadvantages of early versus late intubation are controversial. Also, it should be admitted that the COVID-19 pandemic is an opportunity to learn more about critically ill patients (12, 13).
2. Objectives
The mortality rate resulting from intubation was reported at 15 to 36% (2). Accordingly, it seems that some clinicians delayed intubation. The timing of intubation in COVID-19 patients seems to be challenging. Therefore, we intended to find out how the time of intubation in COVID-19 patients affected survival and which clinical features were connected with mortality.
3. Methods
This cross-sectional study was conducted in the Imam Khomeini Hospital Complex and approved by the Ethical Committee of the Tehran University of Medical Sciences (No. IR.TUMS.IKHC.REC.1399.483). Data were gathered from hospitalized patients (aged 18 to 80) who had laboratory-confirmed SARS-CoV-2 infection and were later admitted to critical care units. A positive real-time reverse transcription-polymerase chain reaction (PCR) test of the nose and pharyngeal swabs confirmed the presence of SARS-CoV-2. All COVID-19 patients who were admitted to the ICU and met the Berlin definition (14) of ARDS, or respiratory distress, were included. All the patients received supplemental oxygen or noninvasive ventilation masks. NIV failure was considered when patients under NIV needed intubation (15).
From the medical records, demographic and baseline information such as age, gender, vital signs, comorbidity conditions, and first laboratory results were acquired. The acute physiological and chronic health evaluation II (APACHE) and sequential organ failure assessment (SOFA) scores were used to assess the severity of the illness. There was no information available on serial ventilatory parameters.
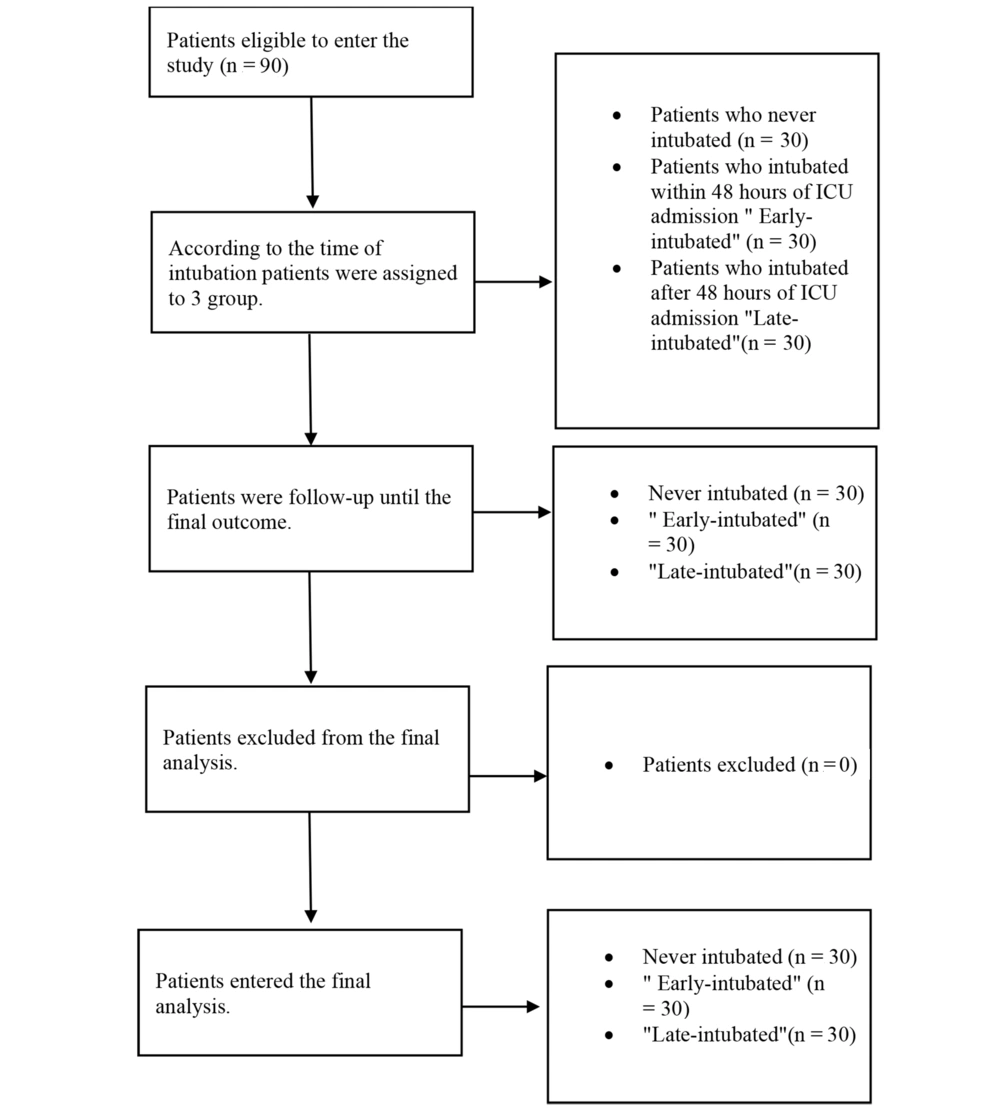
According to the time of intubation, patients admitted to intensive care units were categorized into three subgroups: (1) group 1: Early intubated; (2) group 2: Late intubated; and (3) group 3: Not intubated (Figure 1). Early intubation is defined as intubation within 48 hours of ICU admission, and late intubation is defined as intubation after 48 hours of ICU admission. Endotracheal intubation was based on the clinical judgment of the intensivist/anesthesiologist. We did not have a fixed timing protocol for utilizing NIV. Decision-making about inserting an endotracheal tube was according to the clinician's judgment.
Data for continuous variables were reported as medians, while categorical variables were expressed as numbers and percentages. The Mann-Whitney U test or t-test was employed for continuous variables in bivariate analysis, and Chi-square test or Fisher's exact test was used for categorical variables. To compare variables in the three groups of patients, the ANOVA test or Kruskal-Wallis H test was performed. The multivariate analysis model included factors that differed according to clinical outcomes with a P-value of < 0.05 or were deemed clinically significant. All statistical procedures were performed using SPSS software version 26.
4. Results
A total of 90 patients were included in this study. The average age was 61.1 years, the median BMI was 27.8, and 61.1% of the patients were male. The mortality rate was 57.8%. Besides, 66.7% of the patients experienced NIV failure. The patients’ demographic and clinical characteristics are shown in Table 1.
| Items | Not Intubated (n = 30) | Early Intubated (n = 30) | Late Intubated (n = 30) | P-Value a |
|---|---|---|---|---|
| Ischemic heart disease | 13 | 13 | 20 | 0.113 |
| Neurology disease | 3 | 2 | 2 | 0.856 |
| Endocrine disease | 8 | 9 | 7 | 0.843 |
| Pulmonary disease | 2 | 2 | 4 | 0.578 |
| Diabetes mellitus | 14 | 9 | 14 | 0.317 |
| End stage renal disease or chronic kidney disease | 4 | 6 | 3 | 0.533 |
| Human immunodeficiency virus (HIV) or acquired immunodeficiency syndrome (AIDS) | 2 | 0 | 0 | 0.129 |
| Cancer or malignancy | 2 | 1 | 5 | 0.168 |
| Hypertension | 14 | 16 | 17 | 0.732 |
| Morbid obesity | 2 | 4 | 3 | 0.690 |
| Antibiotic b | 13 | 18 | 20 | 0.171 |
| Dexamethasone b | 25 | 24 | 25 | 0.927 |
| Remdesivir b | 22 | 16 | 15 | 0.139 |
| ReciGen®b | 9 | 8 | 7 | 0.843 |
| Heparin or enoxaparin b | 17 | 12 | 19 | 0.175 |
| Methylprednisolone b | 5 | 5 | 8 | 0.535 |
| Hydroxychloroquine c | 7 | 2 | 2 | 0.075 |
| Losartan c | 10 | 13 | 12 | 0.721 |
| Atorvastatin c | 17 | 20 | 20 | 0.650 |
| Aspirin c | 19 | 19 | 15 | 0.480 |
| Vitamin C c | 16 | 19 | 24 | 0.090 |
| Vitamin D3 c | 15 | 10 | 12 | 0.418 |
| Zinc c | 15 | 16 | 14 | 0.875 |
| Cough | 25 | 26 | 24 | 0.786 |
| Fever | 18 | 22 | 16 | 0.266 |
| Dyspnea | 13 | 26 | 23 | 0.000 |
| Myalgia | 19 | 18 | 20 | 0.866 |
| Rhinorrhea | 14 | 16 | 17 | 0.732 |
Patients’ Demographic and Clinical Characteristics
Among the clinical characteristics, the relationship between dyspnea and stage of intubation was significant, χ2 (2) = 14.412, P = 0.00074. Early-intubated patients were more likely to have dyspnea than late-intubated patients.
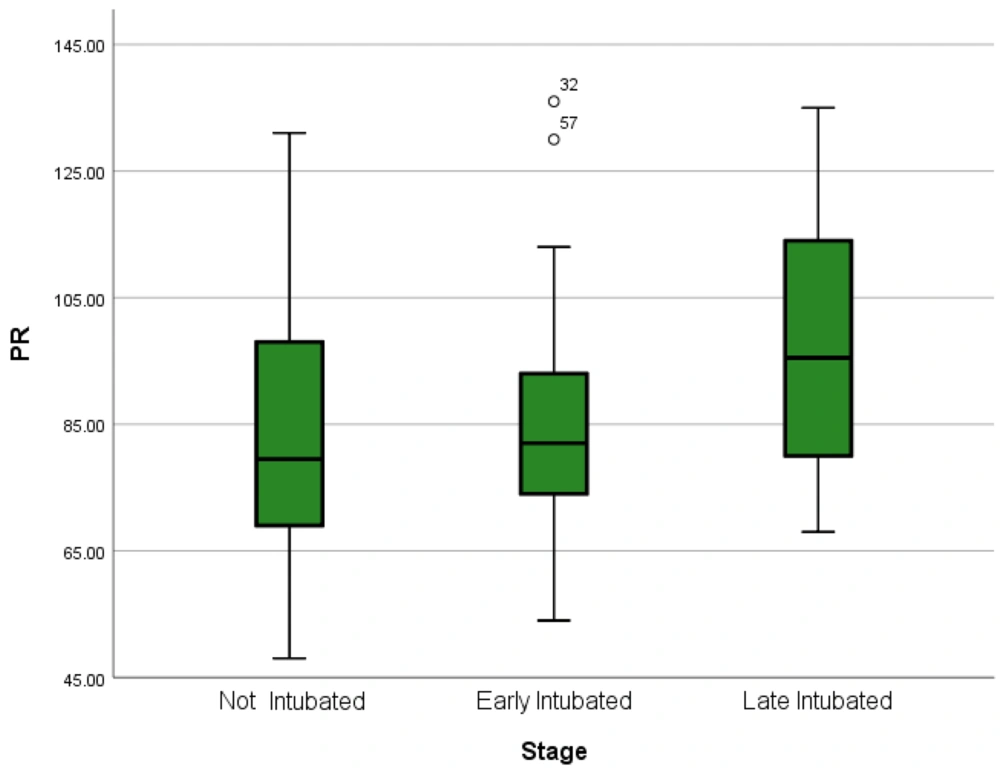
Comparing the median of variables between early- and late-intubated patients using the Mann-Whitney U test showed a statistically significant difference in the patients' heart rate (per minute). Thus, late-intubated patients had a higher mean heart rate than the others (Figure 2). However, other variables did not show statistically significant differences between early- and late-intubated patients (P > 0.05). Then, variables were compared between the three groups of patients using the Kruskal-Wallis H test (Table 2).
| Variables | Not Intubated | Early Intubated | Late Intubated | P-Value | χ2 |
|---|---|---|---|---|---|
| Age | 60 ± 17.21 | 59.26 ± 14.75 | 64.03 ± 14.33 | 0.442 | 1.643 |
| APACHE2 | 15.66 ± 4.51 | 23.60 ± 6.52 | 22.23 ± 5.03 | 0.000 | 28.493 |
| SOFA | 4.43 ± 2.05 | 8.33 ± 2.94 | 8.73 ± 2.46 | 0.000 | 28.815 |
| Day to admission | 9 ± 4.77 | 6.30 ± 3.05 | 7.13 ± 3.26 | 0.030 | 7.008 |
| Day to ICU | 3.33 ± 4.65 | 2.90 ± 3.12 | 2.73 ± 2.80 | 0.841 | 0.347 |
| SPO2 (ICU admission) | 87 ± 7.22 | 82.66 ± 6.91 | 83.26 ± 5.75 | 0.027 | 7.252 |
| Temperature (T) | 36.84 ± 0.67 | 36.78 ± 0.68 | 36.87 ± 0.63 | 0.711 | 0.681 |
| Pulse rate (PR) | 85 ± 21.4 | 85.86 ± 19.74 | 97.27 ± 18.71 | 0.027 | 7.238 |
| Respiratory rate (RR) | 21.46 ± 4.95 | 32.96 ± 7.06 | 31.4 ± 7.65 | 0.000 | 36.703 |
| WBC | 10896 ± 6720 | 12661 ± 7877 | 13112 ± 6099 | 0.257 | 2.738 |
| Hemoglobin | 12.86 ± 3.42 | 12.32 ± 2.50 | 13.46 ± 1.70 | 0.254 | 2.720 |
| Platelet (PLT) | 249500 ± 100501 | 211333 ± 79633 | 224166 ± 107470 | 0.358 | 2.054 |
| CRP | 90.16 ± 56.66 | 109.41 ± 120.58 | 107.96 ± 61.73 | 0.495 | 1.407 |
| LDH | 744 ± 336 | 864 ± 376 | 1025 ± 461 | 0.044 | 6.235 |
| ESR | 67.26 ± 63.73 | 56.20 ± 20.93 | 66.76 ± 27.33 | 0.236 | 2.884 |
| PH | 7.38 ± 0.08 | 7.34 ± 0.11 | 7.35 ± 0.09 | 0.570 | 1.124 |
| PaCO2 | 39.82 ± 8.46 | 45.03 ± 10.63 | 45.87 ± 13.15 | 0.790 | 5.072 |
| PaO2 | 41.52 ± 12.5 | 42.94 ± 13.67 | 42.04 ± 13.25 | 0.995 | 0.010 |
| HCO3 | 23.83 ± 5.07 | 24.06 ± 6.11 | 26.73 ± 5.99 | 0.203 | 3.193 |
| Cr | 1.26 ± 0.68 | 1.43 ± 1.11 | 1.48 ± 1.61 | 0.532 | 1.261 |
| Urea | 45.7 ± 22.56 | 73.6 ± 61.01 | 52..36 ± 35.67 | 0.145 | 3.862 |
| ALT | 46.4 ± 50.86 | 71.83 ± 70.21 | 48.7 ± 22.7 | 0.578 | 1.096 |
| AST | 54.8 ± 39.20 | 83.6 ± 13.6 | 58.3 ± 25.76 | 0.525 | 1.287 |
| ALP | 218 ± 132 | 197 ± 95 | 206 ± 128 | 0.941 | 0.121 |
| Na | 138.56 ± 4.89 | 138.51 ± 5.32 | 138.61 ± 4.64 | 0.896 | 0.221 |
| K | 4.4 ± 0.54 | 5.40 ± 6.74 | 4.25 ± 0.62 | 0.261 | 2.686 |
| Ca | 7.9 ± 0.61 | 7.99 ± 0.74 | 7.89 ± 0.73 | 0.999 | 0.002 |
| P | 3.28 ± 0.93 | 3.64 ± 1.48 | 3.68 ± 1.65 | 0.865 | 0.312 |
| Mg | 2.49 ± 0.58 | 2.35 ± 0.39 | 2.53 ± 0.49 | 0.427 | 1.0703 |
| Bilirubin total (BilT) | 0.92 ± 0.68 | 0.89 ± 0.47 | 0.95 ± 1.02 | 0.889 | 0.235 |
| Bilirubin direct (BilD) | 0.43 ± 0.42 | 0.40 ± 0.28 | 0.58 ± 1.22 | 0.931 | 0.144 |
| Pt | 13.46 ± 1.8 | 13.55 ± 2.02 | 13.57 ± 2.01 | 0.953 | 0.097 |
| INR | 1.08 ± 0.11 | 1.11 ± 0.16 | 1.10 ± 0.16 | 0.910 | 0.188 |
| aPTT | 32.76 ± 8.65 | 36.62 ± 15.13 | 33.93 ± 14.65 | 0.351 | 2.095 |
| CPK | 86.14 ± 120 | 78.96 ± 92.44 | 203.5 ± 298.06 | 0.031 | 6.934 |
| Troponin I | 0.18 ± 0.02 | 0.28 ± 0.43 | 0.22 ± 0.09 | 0.012 | 8.842 |
| Lymphocyte count | 1097 ± 684 | 732 ± 400 | 882 ± 706 | 0.106 | 4.481 |
| Neutrophil count | 8785 ± 5429 | 11265 ± 7593 | 11484 ± 5749 | 0.134 | 4.016 |
| ROX | 4.49 ± 1.12 | 2.77 ± 0.75 | 3 ± 1 | 0.000 | 38.071 |
| Neutrophil/lymphocyte ratio (NLR) | 10.47 ± 7.77 | 17.38 ± 10.94 | 18.89 ± 13.22 | 0.007 | 9.872 |
Comparison of Mean Variables in Three Groups of Patients a
Not-intubated patients had lower APACHE 2 and SOFA scores than the other two groups (P < 0.005). The mean number of days spent from illness to hospitalization was significantly higher (P < 0.05) among not-intubated patients than in late- and early-intubated patients. Patients who were intubated early had a lower mean SPO2 than late- and not-intubated patients (P < 0.05). The mean heart rate was higher in late-intubated patients than in early- and not-intubated patients, and the mean respiratory rate was higher in early-intubated patients than in late- and not-intubated patients. Late-intubated patients had the highest mean lactate dehydrogenase level, followed by patients who were intubated early and those who were not intubated. Mean serum creatine phosphokinase and troponin I levels were higher in late-intubated patients than in early- and not-intubated patients. Early-intubated patients had a lower ROX index than late-intubated patients. Finally, the neutrophil/lymphocyte ratio (NLR) was significantly (P < 0.05) lower in not-intubated patients than in other patients. There was no difference in NLR between early- and late-intubated patients (P > 0.05).
There is a statistically significant association between intubation and clinical outcome [χ2(2) = 42.176, Phi = 68.5%, P < 0.005]. It means that patients who were not intubated had a lower mortality rate than patients who were intubated early or late. However, there were no differences in mortality rates between early- and late-intubated patients.
| Variables | Odds Ratio (95% Confidence Interval) | P-Value |
|---|---|---|
| APACHE 2 | 0.653 [0.427 - 0.999] | 0.050 |
| SOFA | 0.120 [0.024 - 0.600] | 0.010 |
| Intubation | 0.030 [0.002 - 0.443] | 0.011 |
| Overall model | 2.469 | 0.010 |
Results of Logistic Regression
A logistic regression (Table 3) was performed to ascertain the effects of APACHE, SOFA, and intubation on the survival odds of the patients. The logistic regression model was statistically significant, χ2(4) = 107.149, P < 0.0005. The model clarified 93.6% (Nagelkerke R2) of the variance survivals and accurately categorized 96.7% of the cases. The odds of survival were greater for patients who were not intubated than for intubated patients. Increased APACHE and SOFA scores were associated with decreased odds of survival. An odds ratio is a statistical technique used to forecast the likelihood of an event occurring based on a one-unit change in an independent variable while holding all other independent variables constant.
Binary logistic was performed to predict the intubation, and the model was statistically significant, χ2(4) = 33.946, P < 0.0005 (Table 4). The model explained 82.1% (Nagelkerke R2) of the variance survivals and correctly classified 92.2% of the cases.
| Variables | Odds Ratio (95% Confidence Interval) | P-Value |
|---|---|---|
| APACHE 2 | 1.559 [1.194 - 2.035] | 0.001 |
| RR | 1.101 [1.013 - 1.196] | 0.000 |
| NLR | 1.1.1 [1.013 - 1.196] | 0.024 |
| IHD | 15.612 [1.609 - 151.48] | 0.018 |
| Overall model | 0.09 | 0.000 |
Predictors of Intubation in Critically Ill COVID-19 Patients
It is vividly appeared that APACHE 2 (P = 0.001), RR (P = 0.000), NLR (P = 0.024), and IHD (P = 0.018) were significant predictors of intubation. Patients with higher scores of APACHE 2, higher respiratory rates, and a higher neutrophil to lymphocyte ratio are more likely to be intubated. Also, the odds of intubation in the ICU are roughly 15 times greater for patients with an ischemic heart disease background than for others. However, age, gender, other background diseases, receiving dexamethasone/Remdesivir before ICU admission, and receiving vitamin D and C were not statistically significant predictors of intubation.
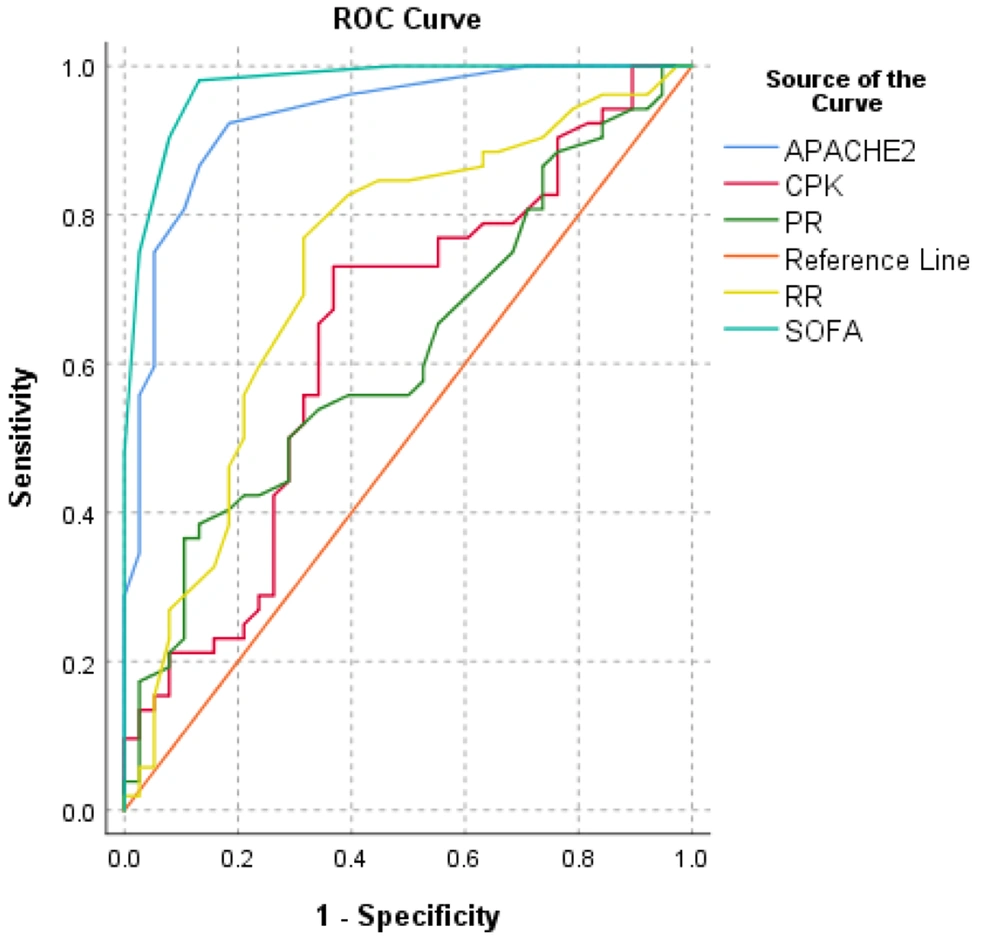
The receiver operating characteristic (ROC) curve was utilized to assess the diagnostic accuracy (mortality) and the best prediction threshold for COVID-19 intensification (Table 5). The ROC curve analysis showed that APACHE2 score (AUC = 93.1%, P < 0.005), SOFA score (AUC = 97.4%, P < 0.005), respiratory rate (AUC = 72.9%, P < 0.005), pulse rate (AUC = 61.7.9%, P < 0.05), and creatine phosphokinase (AUC = 63.9%, P < 0.05) were predictors of mortality. The prediction efficiency is shown in Figure 3.
| Test Result Variable(s) | Area | Asymptotic Sig. | 95% Confidence Interval |
|---|---|---|---|
| APACHE2 | 0.931 | 0.000 | [0.878 - 0.984] |
| SOFA | 0.974 | 0.000 | [0.947 - 0.982] |
| PR | 0.617 | 0.048 | [0.501 - 0.733] |
| RR | 0.729 | 0.000 | [0.620 - 0.838] |
| CPK | 0.639 | 0.021 | [0.521 - 0.757] |
ROC Curve Analysis of Clinical Data
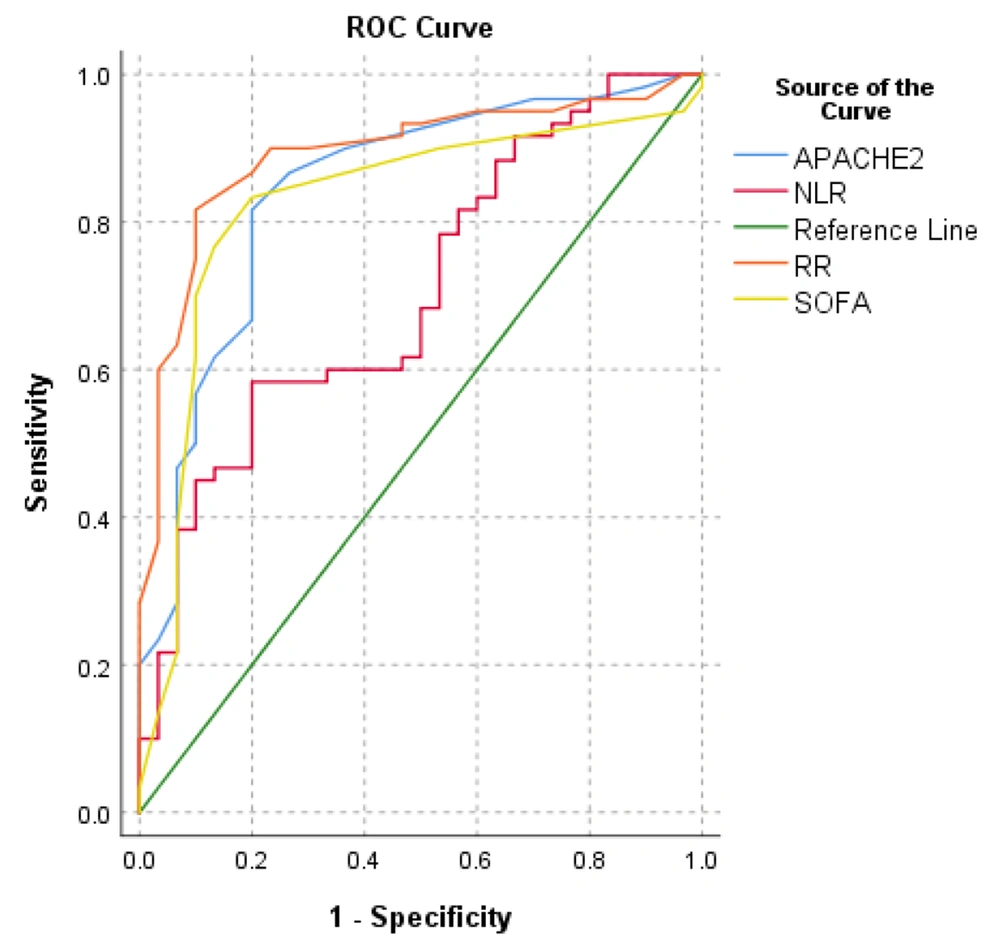
The diagnostic accuracy (intubation) and appropriate prediction threshold of COVID-19 intensification were investigated using the ROC curve (Table 6). The ROC curve analysis revealed that the ROX index (AUC = 89.9%, P < 0.005), APACHE2 score (AUC = 84.4%, P < 0.005), SOFA score (AUC = 82.5%, P < 0.005), respiratory rate (AUC = 89.1 %, P < 0.005), and NLR (AUC = 70.4 %, P < 0.005) were statistically significant in predicting mortality. Figure 4 depicts the prediction efficiency.
| Test Result Variable(s) | Area | Asymptotic Sig. | 95% Confidence Interval |
|---|---|---|---|
| ROX | 0.899 | 0.000 | [0.830 - 0.967] |
| APACHE2 | 0.844 | 0.000 | [0.755 - 0.933] |
| SOFA | 0.825 | 0.000 | [0.727 - 0.923] |
| RR | 0.891 | 0.000 | [0.820 - 0.962] |
| NLR | 0.704 | 0.000 | [0.592 - 0.815] |
ROC Curve Analysis of Clinical Data for Predicting Intubation
5. Discussion
Our study was conducted to examine how the timing of intubation in COVID-19 patients may have survival benefits. We investigated clinical factors linked to outcomes in critically ill COVID-19 patients with ARDS. We looked at which clinical factors are linked to outcomes in critically ill COVID-19 patients with ARDS. Of the patients, one-third were intubated within 48 hours of ICU admission (early) and one-third after 48 hours of ICU admission (late). Studies on the mortality rate of COVID-19 patients in ICUs reported it to range from 25.7% (16) to 36.0% (2), which is lower than our findings. However, because inclusion criteria vary in different studies and some patients in our research had a poor prognosis, the real fatality rate might be more significant.
We found that the NIV failure rate was 66.7% for all the patients. However, it was previously reported to range from 5% to 60% in COPD and acute respiratory failure (ARF) (17, 18). Besides, Nicolini et al. (19) reported NIV failure in 20% of community-acquired pneumonia (CAP). Our results showed a high rate of NIV failure compared to previous studies. Also, Menzella et al. (20) reported the NIV failure rate of 51.9% (41 out of 79), which is close to our reported rate (60 out of 90). However, Mukhtar et al. (21) reported the NIV failure rate at 26% (13 out of 49 patients) in their study. It should be mentioned that their sample size was smaller than in the present study. Accordingly, NIV failure seems more common in COVID-19 patients than in COPD, CAP, and ARF patients. It should be considered that patients under NIV have different needs that should be addressed properly (22).
The main findings of this study are aligned with the study of Lee et al. (4). They advocated that in-hospital mortality did not differ significantly between early- and late-intubated patients. Furthermore, the conclusion of a meta-analysis (6) indicated that the timing of intubation seems to have no direct effect on mortality or supposed comorbidity. However, it is fair to consider different ways of practicing that can affect the time of intubation and, consequently, the final outcome. Decision-making regarding the insertion of an endotracheal tube should be according to the patient's circumstances and the clinical judgment of the practitioner (13, 23).
Although we did not find any significant differences (survival benefits) between early- and late-intubated patients, some pieces of evidence and clinician reports are in favor of early intubation (invasive ventilation). First of all, Gattinoni et al. (10) hypothesized that we could consider an early intubation strategy for P-SILI prevention. Second, it is suggested that early intubation can reduce the risk of contamination to healthcare providers (7). Thirdly, some criticized late intubation for the supposed dire consequences. Late-intubated patients are reported (24) to have low lung compliance or more detrimental ventilatory ratios with conceivably higher mortality. It can result from self-induced lung injury or the nature of severe progressive inflammation. In contrast, some papers criticized the liberal use of early intubation. Tobin et al. (25) believe that PSILI is a new term, and there are no exact definitions for it. They advocated that some severe hypoxemic COVID-19 patients with normal lung compliance did not develop dyspnea. Indeed, this occurs because the amount of hypoxemia is not low enough to elicit increased respiratory motor effort and the concomitant PaCO2 levels dampen the hypoxic response. Although there is insufficient data to advocate late (delayed) intubation, it is a hindsight decision (11). Undoubtedly, different practices have different effects; thus, early or late intubation may have a different effect on COVID-19 survival, although there is insufficient evidence to advocate either of them. It should be clarified which strategy is used to overcome the other one.
A review (26) recommended that intubation and mechanical ventilation are multifactorial decisions and thus should not be done according to a single parameter such as decreased oxygen saturation or lung involvement on a CT scan. It is also suggested that patients with moderate to severe hypoxemia should receive supplemental oxygen with HFNC, and an awake prone position for a short trial can be considered.
Our statistical model results in terms of predictors of intubation revealed that APACHE 2 scores, NLR, RR, and history of ischemic heart disease were appropriate predictors of intubation in critical care settings. De Vita et al. (27) suggested that in COVID-19 patients who needed continuous positive airway pressure (CPAP), higher age, LDH, and change in PaO2/FiO2 ratio after initiating CPAP could be independent predictors of intubation. These results are not controversial and can broaden our horizons to include the prediction of intubation of COVID-19 patients. Mueller et al. (28) revealed that in COVID-19 inpatients with stable CRP levels, rising CRP levels predicted intubation. As a result, increasing CRP within the first 48 hours of hospitalization predicts respiratory deterioration better than initial CRP levels or ROX indices. However, we did not screen the CRP level of the patients. According to Suliman et al. (29), the ROX index might be a straightforward, noninvasive approach for predicting the discontinuation of high-flow oxygen treatment and can monitor progress and the risk of intubation in COVID-19 pneumonia patients. The ROX index was found in this study to be one of the most sensitive and competent techniques for predicting intubation. According to Tatum et al. (30), NLR is a predictor of endotracheal intubation upon hospitalization and an independent predictor of the risk of mortality in SARS-CoV-2 patients on subsequent hospital days. Our findings also showed that NLR could be used as one of the predictors of endotracheal intubation. The SOFA score and the ROX index may both be used to identify patients who are more likely to require intubation (31), which is consistent with our findings.
APACHE 2 and SOFA scores are two of the most essential prognosis-predicting tools in critical settings. Our study showed that both of these scores are appropriate tools to assess the prognosis of COVID-19 patients who are admitted to ICUs. Han et al. (32) also proved the importance of these two scores in their research. Also, the findings indicated that respiratory rate and heart rate could be used to evaluate the prognosis of patients. Furthermore, Huang et al. (33) proposed that an elevated respiratory rate appeared to be related to a patient's prognosis. Creatine phosphokinase has been linked to poor outcomes and can be used as a prognosis factor. This finding is also aligned with Orsucci et al.’s findings (34).
5.1. Limitations
There are various limitations to our study. This is a prospective study with 30 patients in each group and a medium sample size. As a result, some variables deviated from the normal distribution. The laboratory samples were taken in a critical situation that may have resulted in sampling error or false results. The ventilatory variables such as lung compliance and resistance, tidal volume, and positive end-expiratory pressure were not recorded for mechanically ventilated patients. These variables can help predict liberation (weaning process), complications (like acute kidney injury), and mortality. We followed patients until they were discharged or expired, and we did not consider hospital readmission or in-home mortality. However, our data aligned with previous studies to improve our knowledge of COVID-19 critical care.
5.2. Conclusions
There were no statistically significant differences in total mortality between early- and late-intubated patients. Higher respiratory rates (tachypnea) can indicate early intubation. APACHE 2 scores, NLR, RR, and history of ischemic heart disease are some of the appropriate predictors of intubation. Also, the ROX index is one of the most sensitive and capable tools for predicting intubation. Intubation status, APACHE, and SOFA scores are potent predictors of in-hospital mortality. The intubation strategy for COVID-19 is rather according to clinicians' decision.