1. Background
Knee osteoarthritis is a prevalent joint disorder, and various medical, non-medical, and surgical methods have been offered for its treatment (1-4). Total knee arthroplasty (TKA) is one of the most common orthopedic surgeries, which reduces pain, improves function, and enhances the quality of life of patients with knee osteoarthritis. However, about 20% to 35% of patients undergoing this surgery are still dissatisfied, mainly due to chronic residual pain (5). Inadequate management of postoperative pain leads to prolonged hospitalization, increased cost of care, delayed rehabilitation, and increased risk of postoperative complications (6). Therefore, various pharmacological and non-pharmacological methods have been used to control acute and chronic pain and perioperative pain (7-10).
So far, there have been studies on the effects of gabapentinoids, duloxetine, dexmedetomidine, ketamine, lidocaine, magnesium, and regional blocks (11-17). Despite the advances in pain management, opioids are still commonly used to treat these conditions (18). Duloxetine is a norepinephrine and serotonin reuptake inhibitor widely prescribed for major depressive disorders, generalized anxiety disorders, diabetic peripheral neuropathy, and chronic musculoskeletal pain (12). Duloxetine increases norepinephrine and serotonin and ultimately improves the descending inhibitory effects in the central nervous system. The analgesic effects of duloxetine are independent of its antidepressant effects, and it has similar analgesic effects in both groups of depressed and non-depressed patients. In some studies, the analgesic effects of duloxetine in chronic musculoskeletal pain have been observed, but its impact on perioperative TKA pain control is debatable (19).
Pregabalin is a structural analog of GABA and is an anticonvulsant drug that selectively affects the process of pain transmission through nociceptors by inhibiting calcium channels. Compared to gabapentin, pregabalin has better oral absorption and bioavailability. Recently, pregabalin has been considered an adjuvant drug in the management of neuropathic and postoperative pain, and several studies have shown that it reduces acute pain and postoperative opioid consumption following some surgeries (20). Its perioperative administration may help prevent chronic postoperative pain. Studies report conflicting results about pregabalin's effects, which is a matter of controversy (21). There are studies on the effectiveness of duloxetine and pregabalin in controlling pain after TKA, but no comparative study has been conducted to determine which of these two drugs is more effective in controlling postoperative pain.
2. Objectives
This clinical trial aimed to compare the effect of perioperative administration of pregabalin and duloxetine on pain management after total knee arthroplasty.
3. Methods
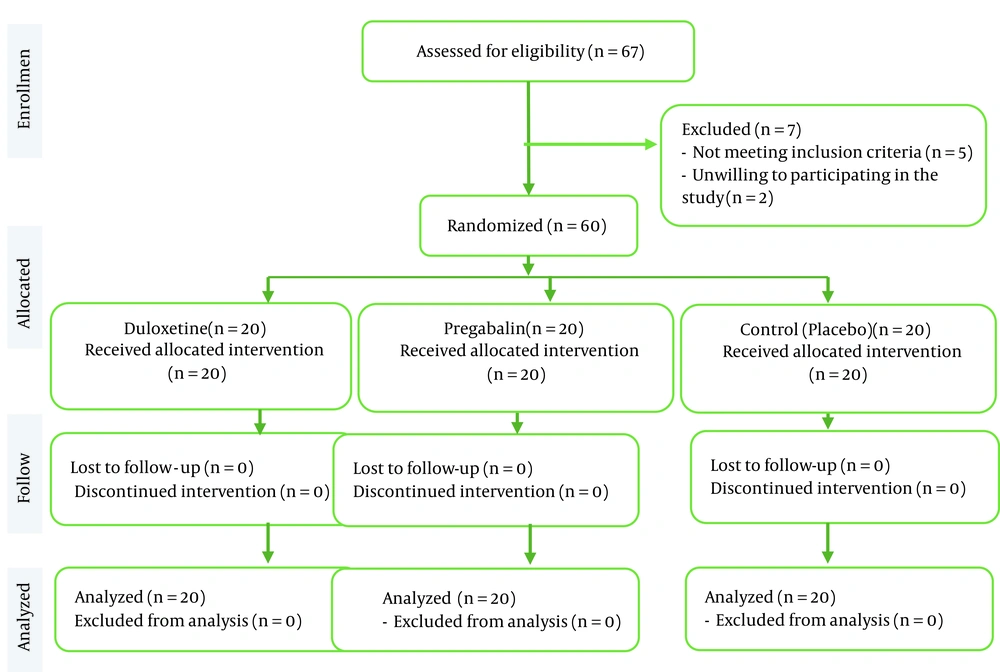
After the approval of the Ethics Committee of the Iran University of Medical Sciences (code: IR.IUMS.FMD.REC.1398.378) and the Iranian Registry of Clinical Trials (IRCT code: IRCT20190719044276N1), in this randomized, double-blind placebo control clinical trial, TKA candidate patients were studied according to the CONSORT guidelines (Figure 1). Eligibility criteria included age 40 to 75 years and ASA I-II candidates for TKA under spinal anesthesia. Patients with lower extremity neuropathic pain, drug abuse, psychological disorders, drug sensitivity, severe cardiac, renal, or hepatic diseases, or refusing regional anesthesia were excluded. Allocating patients to the studied groups was done by a blinded collaborator not involved in study procedures. Patients and clinical researchers were also blinded to the groups and interventions.
Our primary outcome was the VAS score at 48 hours. Secondary outcomes were analgesic use at 48 hours, time to first analgesic request, and the WOMAC scores six months after surgery compared to the preoperative score.
The sample size of 60 patients (i.e., 20 patients per group) was determined based on the sample size calculation formula. The maximum sample size was calculated by considering the confidence level of 95% and a power of 90% using the following formula (including 15% attrition):
Patients were divided into three equal groups (n=20) according to the block randomization method. Group A received pregabalin 75 mg, group B duloxetine 30 mg, and group C placebo. Intervention (prescription of drugs) was performed in all groups three times, 90 minutes before surgery, then 12 and 24 hours after surgery. After obtaining written informed consent, all patients were subjected to standard monitoring, including pulse oximetry, ECG, and non-invasive blood pressure. Ringer's solution (500 mL) was infused. In the sitting position, a 25G Quincke spinal needle was inserted into the intrathecal space through the L4 - 5 intervertebral space. After the free flow of CSF, bupivacaine 0.5% (AstraZeneca, Austria) 10 mg and fentanyl 25 μg were slowly injected. After obtaining the appropriate level of anesthesia, the surgery would begin. If spinal anesthesia was unsuccessful, general anesthesia would be performed, and the subject would be excluded. During the first postoperative day, if the VAS score was more than 3 in every four visits, i.v. paracetamol 1 g (Apotel, Uni-pharma Co., Greece) was administered. The VAS scores were recorded 6, 12, 24, and 48 hours after surgery, and the WOMAC scores before and six months after surgery. The dose of paracetamol consumed 6, 12, 24, and 48 hours after the surgery and the first analgesic request time were also investigated.
3.1. Data Analysis
Patient data were analyzed using SPSS version 22 statistical software. Central indices (mean and SD) were used to report quantitative parameters. Frequency (%) was used to report qualitative parameters. The normality of the distribution of the parameters was determined using the Kolmogorov-Smirnov test. Considering the independence of the study groups, with the assumption of normal distribution of the variables, ANOVA was used to compare more than two groups. The Kruskal-Wallis non-parametric test was used to compare variables in three groups. The chi-square test was used to analyze qualitative variables in groups. A repeated measurement test was used to compare the index at different times during the treatment. A P value < 0.05 was considered statistically significant.
4. Results
In this clinical trial, 67 patients were screened for inclusion. Five patients were excluded because they did not meet the inclusion criteria, and two were excluded because they did not consent to participate in the study. Finally, 60 patients were studied in three equal groups (n = 20). During the intervention and follow-up, no patient had to be excluded from the study (Figure 1).
Demographics and results are shown in Table 1. No significant difference was observed in demographic variables among the three groups at the beginning of the study. The mean VAS score at all times in the three groups and the time to first analgesic request were statistically significantly different between groups A and B, on the one hand, and group C on the other, but there was no significant difference between groups A and B. No significant difference was observed in the WOMAC score between the three groups.
| Variables | A | B | C | P Value |
|---|---|---|---|---|
| Age (y) | 66.8 ± 7.0 | 67.6 ± 4.3 | 65.5 ± 6.4 | 0.18 |
| Sex; No. (%) | 0.15 | |||
| Male | 3 (15) | 1 (5) | 0 (0) | |
| Female | 17 (85) | 19 (95) | 20 (100) | |
| Surgery duration (h) | 2.1 ± 0.5 | 2.2 ± 0.4 | 2.1 ± 0.3 | 0.62 |
| VAS (h) | ||||
| 6 | 5.0 ± 1.0 | 4.8 ± 0.9 | 5.2 ± .08 | 0.001 |
| 12 | 3.2 ± 0.9 | 3.5 ± 0.8 | 4.9 ± 1.0 | 0.001 |
| 24 | 2.7 ± 1.1 | 2.4 ± 1.0 | 3.8 ± 0.9 | 0.017 |
| 48 | 2.1 ± 0.9 | 1.9 ± 0.8 | 2.7 ± 1.0 | 0.01 |
| First analgesic request time (h) | 16.9 ± 1.7 | 17.1 ± 2.2 | 15.4 ± 1.6 | 0.001 |
| Paracetamol (g) | ||||
| 6 h | 0.7 ± 0.4 | 0.9 ± 0.5 | 1.0 ± 0.5 | 0.033 |
| 12 h | 0.6 ± 0.5 | 0.8 ± 0.4 | 0.9 ± 0.6 | 0.001 |
| 24 h | 0.5 ± 0.1 | 0.6 ± 0.3 | 1.2 ± 0.6 | 0.03 |
| 48 h | 0.6 ± 0.2 | 0.6 ± 0.1 | 1.6 ± 0.5 | 0.001 |
| WOMAC | ||||
| Before | 63.2 ± 5.1 | 63.5 ± 3.2 | 62.8 ± 4.2 | 0.89 |
| 6 months | 26.6 ± 3.9 | 25.9 ± 3.9 | 26.3 ± 3.2 | 0.82 |
The mean consumption of i. v. paracetamol in the first six hours was significantly lower in group A than in groups B and C (P = 0.03). In contrast, no significant difference was reported between groups B and C (P = 0.11). In the first 12 hours, it was lower in group A than in groups B and C (P = 0.033), but no significant difference was observed between groups B and C (P = 0.11). Twenty-four hours after surgery, it was higher in group C than in groups A and B (P = 0.03). But there was no significant difference between groups A and B (P = 0.19). Forty-eight hours after surgery, it was significantly higher in group C than in groups A and B (P = 0.001), while no significant difference was reported between groups A and B (P = 0.58). The trend of paracetamol consumption over time in the three groups was statistically different (P = 0.001). No complications were observed with administering these two drugs in the patients.
5. Discussion
In this study, oral perioperative pregabalin and duloxetine in knee arthroplasty significantly reduced postoperative pain score and analgesic consumption. However, a reduction in VAS by less than 20 mm or paracetamol dose by less than 1 g was probably not clinically significant but did not affect knee movement status (WOMAC score) six months after surgery.
Since pain after TKA can have both nociceptive and neuropathic origins, using effective drugs to treat neuropathic and nociceptive pain can control knee arthroplasty pain (22, 23). Duloxetine can play the role of desensitizing the CNS in patients with central sensitization and thus be effective in controlling neuropathic pain after TKA (24). On the other hand, pregabalin can selectively affect the process of pain transmission through nociceptors and be efficient in managing pain after arthroplasty (25). There are many studies on the effectiveness of these two drugs in controlling pain after TKA, but no study has been conducted to compare these two drugs in controlling pain after TKA (21, 24).
There are several studies with different doses of perioperative pregabalin for pain management after knee arthroplasty, but there are few studies with a dose of 75 mg. A high perioperative dose of pregabalin leads to optimal pain management after TKA and improves knee range of motion after surgery. In the study of Buvanendran et al., pregabalin 300 mg was prescribed before TKA and pregabalin 150 mg for 14 days after surgery (26). Patients were screened for neuropathic pain three and six months after surgery. Secondary outcomes included postoperative recovery and rehabilitation measures, including knee range of motion, opioid use, postoperative pain scores, sleep disturbance, discharge time, and postoperative complications. Their study showed that postoperative pregabalin was associated with less analgesic consumption and improved range of motion during the first four weeks of rehabilitation. It also reduced the incidence of chronic neuropathic pain. However, it was associated with a higher risk of early postoperative sedation and confusion at tested doses. Similar to our study, Jain et al. investigated the effect of pregabalin 75 mg compared with a placebo on pain management after TKA (27). This drug was prescribed before the operation and continued twice a day for 48 hours after arthroplasty. Their study showed that pregabalin significantly reduced mean pain score and analgesic consumption during the first 48 hours after surgery. The results of our study are also consistent with their research, as significant reductions in pain, paracetamol consumption, and time to the first analgesic request were observed in the first 48 hours after the surgery.
In addition to pain and analgesic consumption, knee movement status (WOMAC score) was also examined in our study. However, some studies have contradicted these results. In the YaDeau et al.’s study, pregabalin (0, 50, 100, or 150 mg) was administered from before to two weeks after surgery (28). They stated that pregabalin did not have beneficial analgesic effects, and their results did not support the preoperative administration of pregabalin for TKA patients. In general, due to contradictory results in prescribing pregabalin in TKA pain management, it is recommended to conduct more studies with various doses.
The use of duloxetine in controlling TKA pain is based on the logic that part of the pain after TKA may have a neuropathic origin that occurs due to central sensitivity. Therefore, pain can be controlled by desensitizing the CNS with drugs such as duloxetine. Evaluating the effect of duloxetine on the amount of morphine needed after knee arthroplasty, Ho et al. showed that the administration of duloxetine after surgery could reduce the need for morphine in the first 48 hours after surgery, but it did not affect pain and side effects (24). YaDeau et al. administered duloxetine 60 mg daily for 14 days to evaluate its impact on subacute pain after TKA. Their study showed no reduction in pain at rest or during knee flexion, but opioid use and nausea were significantly reduced (29).
Patients with central sensitization have more severe pain after TKA, and, as a result of taking more opioids, so the analgesic effect of perioperative duloxetine in knee arthroplasty can be significantly stronger in these patients. Koh et al. investigated the effects of duloxetine on pain scores after TKA in patients with previous central sensitization. In their study, duloxetine reduced postoperative pain and improved the quality of postoperative recovery without increasing side effects (30). Based on previous studies, for patients with central sensitization before TKA, perioperative duloxetine to the multimodal analgesia protocol may reduce postoperative pain and opioid consumption and improve the quality of recovery without increasing the risk of complications (24, 30). Therefore, oral duloxetine is effective when central sensitization exists before the surgery, i.e., when the origin of knee osteoarthritis pain is neuropathic, while it may be ineffective in other patients. Therefore, the results of our study regarding the successful postoperative pain management after TKA following duloxetine may indicate that most of our patients in the duloxetine group had central sensitization before the surgery. However, there are currently insufficient clinical data to recommend the routine use of duloxetine in patients with central sensitization to improve pain after TKA.
As the results were statistically significant for both medications, but the improvement was too small to be clinically significant, the next step might be to set up a study using a combination of pregabalin and duloxetine to see whether the combined effect would be clinically significant.
5.1. Conclusion
Perioperative oral pregabalin and duloxetine similarly reduced pain and the need for analgesic within 48 hours after TKA in a statistically significant but not clinically significant fashion but did not affect knee mobility after six months. The effects of these two drugs on the amount of pain, time, and analgesic consumption, as well as the knee's movement status, were similar. According to the mechanism of action of duloxetine, it is more logical to prescribe it to patients with neuropathic pain, where it can play a role in desensitizing the central nervous system. Conversely, it is more reasonable to prescribe pregabalin for cases other than central sensitization. As most patients seem to have both neuropathic and nociceptive pain, a multimodal regimen combining both medications might be more effective than each alone, but this hypothesis remains to be investigated.