1. Background
Obesity is one of health care practitioners' most serious health problems. It is defined as a body mass index (BMI) of more than 30 kg/m2, whereas those with a BMI of greater than 35 kg/m2 are classified as morbidly obese, and those with a BMI of greater than 55 kg/m2 as super morbid obese (1). The number of obese people receiving bariatric or non-bariatric surgery continues to rise. Obesity-related pathophysiological alterations predispose these individuals to perioperative problems shortly after general anesthesia (GA) induction. Atelectasis occurs, resulting in a decrease in the ventilation-perfusion ratio and pulmonary compliance (2).
Due to the buildup of perithoracic and abdominal fat, morbid obesity may cause a restrictive condition. GA and supine posture both decrease lung capacity and functional residual capacity (FRC), encouraging the development of atelectasis, altering the ventilation/perfusion ratio, and raising the pulmonary shunt (3).
Since 1994, video-laparoscopy has been utilized to perform bariatric surgery, which is a less intrusive treatment that results in a lower rate of early and late problems as compared to conventional approaches (4). Bariatric surgery's goal is to lower the capacity of the stomach cavity, resulting in the development of satiety following the absorption of a small amount of food. To achieve this early satiety, a tiny gastric pouch is combined with a restricted gastric outlet, referred to as a gastric sleeve. As an alternative, malabsorptive surgeries such as Roux-en-Y gastric bypass are performed. These treatments not only restrict the size of the stomach but also shorten the intestine length (5).
The lung recruitment maneuver (RM) has been utilized to increase gas exchange during anesthesia, which comprises pulmonary inflations and continuous use of positive end-expiratory pressure (PEEP) in patients having laparoscopic bariatric surgery (6). The purpose of RM is to reopen collapsed alveolar units, increase lung area available for gas exchange, and improve arterial oxygenation in patients undergoing abdominal, thoracic, or laparoscopic surgery, as well as in patients at risk of developing moderate degrees of lung injury following surgery (7). When utilized to avoid pulmonary problems during laparoscopic bariatric surgery, RM is a safe and successful method, as demonstrated by the best postoperative spirometric values and radiographic results (8).
Despite the less invasive nature of laparoscopic bariatric surgery, postoperative discomfort can range from mild to severe. Controlling pain in morbidly obese patients might be difficult due to an increased susceptibility to opioid-induced respiratory depression (9).
When combined with conventional analgesic techniques, trocar insertion site local anesthetic infiltration, and systemic analgesia, transversus abdominis plane (TAP) block has been shown to improve pain-related outcomes following upper and lower abdominal surgical procedures and to decrease opioid consumption following laparoscopic bariatric surgery (10).
2. Objectives
This study aimed to evaluate the impact of RM and TAP block performed during laparoscopic bariatric surgery on spirometry, oxygenation, hemodynamic variables, opioid requirements, and pain score assessed after surgery.
3. Methods
This prospective randomized open-label controlled clinical trial was conducted on 80 obese patients of both sexes with ages ranging from 21 to 55 years, ASA class II or III, and BMI between 35 and 50 kg/m2 who were scheduled for elective laparoscopic bariatric surgeries under general anesthesia (GA) [e.g., laparoscopic gastric bypass, and laparoscopic sleeve gastrectomy (LSG)]. The trial was conducted after receiving approval from the Anesthesiology Department Scientific and Ethical Committee (FMASU M D 99a/ 2018-2022), registration on Pan African Clinical Trials Registry (PACTR202203570043004), and getting informed written permission. The study was done from August 2018 to October 2019 at Ain Shams and Helwan University Hospitals in Egypt.
Exclusion criteria were major obstructive and restrictive pulmonary diseases [< 50% of predicted values for pulmonary function tests (PFT) variables], advanced renal or hepatic disease, patients needed postoperative ventilatory support, drug or alcohol dependence, and contraindication to peripheral nerve block (allergy to local anesthetics, infection at the site coagulopathy).
3.1. Randomization
Patients were randomly assigned to four equal groups using computer-generated randomization numbers. Another investigator opened the sealed packet (who had no other roles in the trial). All patients had received a standardized postoperative analgesia regimen. Group, I served as a control group, group II received TAP block after intubation before surgical incision, group III received RM after intubation and after pneumoperitoneal exsufflation, and group IV received RM after intubation and after pneumoperitoneal exsufflation and TAP block after intubation and before surgical incision. The study was open-label due to the different techniques used in each group.
3.2. Preoperative Assessment and Preparation
Routine preoperative assessment was done in all patients on the day before the operation, including history, clinical examination, routine laboratory investigations, chest X-ray, electrocardiogram (ECG), ABG, PFTs, and echocardiography if indicated. Additional investigations were done according to the patient’s medical condition. The spirometry used was Spirox© (Meditech Equipment Co., Ltd., Belgium).
Pre-medication was given to every patient of the four groups in the form of midazolam (2 to 3 mg IV) together with ranitidine (50 mg IV) and metoclopramide (10 mg IV) on arrival to the operative room after establishing peripheral intravenous access under complete aseptic conditions.
3.3. Monitoring
Basic monitoring: including ECG, pulse-oximetry (SpO2), non-invasive blood pressure (NIBP), and EtCO2, was applied to all patients, starting before anesthesia until the end of surgery and then recovery.
Intraoperative hemodynamic measurements for all patients included heart rate (HR), mean arterial blood pressure (MAP), EtCO2, SpO2, and arterial blood gases (ABG). Postoperative hemodynamic measurements included HR, MAP, SpO2, respiratory rate, and ABG for all patients.
3.4. Anesthetic Techniques
Vital signs were constantly monitored until anesthesia was induced. GA was induced intravenously with 1 - 2 ug/kg fentanyl of IBW, 100 mg lidocaine, and 2 mg/kg propofol of IBW + 40% weight excess. Rocuronium, 0.6 mg/kg of real body weight, was used to assist tracheal intubation. Anesthesia was maintained with inhaled isoflurane at a concentration of 1.5 - 2% in a combination of air and oxygen with a FiO2 of 0.8. IV rocuronium was used to maintain muscle relaxation and was titrated to maintain a train of four of 0 until the procedure was completed.
Prior to induction, vital capacity maneuvers with 100% O2 and a 10 cm H2O CPAP in the ramp position were used to acquire preoxygenation and denitrogenation. The lungs were ventilated using volume-controlled ventilation with TV following tracheal intubation. In all patients, 6: 8 mL/kg of IBW and respiratory rate were adjusted to maintain end-tidal CO2 between 35 and 45 mmHg, and 6: 8 cm H2O PEEP was used.
Two RMs consisting of maintaining the airway pressure at 40 cm H2O for 40 seconds (11) were conducted in patients assigned to the RM (group III and IV) following tracheal intubation and pneumoperitoneal exsufflation.
TAP block was administered immediately after induction of anesthesia and prior to surgical incision in patients assigned to groups II and IV. The ultrasound machine used was GE© Vivid S5. Using an oblique subcostal approach, under sterile conditions, a high-frequency linear array transducer was positioned inferior and parallel to the costal margin in a mediolateral orientation. Immediately lateral to the linea semilunaris, the external oblique, internal oblique, and TA muscles were detected. A 22-G, 80-mm, short bevel echogenic needle with a short bevel was inserted medially and parallel to the US beam until the tip was positioned between the internal oblique fascia and the TA muscle layer. Each side received thirty ml of 0.25% bupivacaine with 1: 200,000 epinephrine.
3.5. Postoperative Assessment
At the end of the surgery, awake extubation was done in a semi-sitting position after reversal of muscle relaxation with neostigmine when the patient followed verbal commands, sustained head lift or hand grasp for 5 seconds, and achieved VT of more than 6 mL/kg and respiratory rate of fewer than 35 breaths/min, with stable hemodynamics (12). Then, the patient was transferred to the postanesthesia care unit (PACU). All patients received a standardized postoperative analgesia regimen consisting of paracetamol 1 g/6 h IV and morphine 1mg increments IV (13) in order to achieve a clinical target of 4/10 or lower on a numerical rating scale (NRS) for pain. Patients who needed ventilatory support were excluded from the study. Arterial blood gases and spirometry were done 24h after the operation.
3.6. Measurements
Spirometric values (FVC, FEV1, FEV1/FVC) preoperative and 24h postoperative, oxygenation (PaO2/FiO2 ratio preoperative, intraoperative, postoperative), hemodynamics [(MAP and HR) pre-, intra- and postoperative], total consumption of morphine in 1st 24h postoperative, pain severity score using NRS at 1, 2, 4, 6, 12 and 24h postoperatively, duration of surgery were recorded.
3.7. Sample Size Calculation
The sample size calculation was done by PASS software (version 11.0; NCSS PASS, UT, USA). According to a previous study (14), the mean (± SD) change of FVC was -1 ± 0.5 in the control group and -0.84 ± 0.09 in the TAP group. The expected mean change of FVC is -1.1 and -0.6 for the RM group and RM + TAP group. The sample size was based on the following considerations: 95% confidence limit, 80% power of the study, group ratio 1: 1 and two cases were added to each group to overcome dropout. Therefore, we recruited 20 patients in each group.
3.8. Statistical Analysis
IBM SPSS software was used to analyze the data (statistical program for social science version 21). The data were gathered, normality checked and statistically examined. The mean and standard deviation were used to describe quantitative parametric variables, the median and inter-quartile range (IQR) were used to describe non-parametric variables, and frequency was used to describe qualitative variables. When comparing more than two groups, analysis of variance (ANOVA) with a post-hoc test is used for parametric data, while the Kruskal-Wallis test is used for non-parametric data. In terms of qualitative factors, the chi-square test was performed to compare the groups. The Student t-test was employed in parametric data to compare two groups in terms of quantitative variables. statistical significance was defined as a P value < 0.05.
4. Results
In this study, 107 patients were assessed for eligibility. Twenty did not meet the criteria, and seven refused to be included. The remaining 80 patients were randomly allocated into four groups (20 patients in each). All of them were statistically analyzed and followed up (Figure 1).
Patients' characteristics parameters (age, sex, duration of surgery, and BMI) were insignificantly different among the four groups (Table 1).
| Variables | Group I (control) (n = 20) | Group II (TAP) (n = 20) | Group III (RM) (n = 20) | Group IV (RM + TAP) (n = 20) | P Value |
|---|---|---|---|---|---|
| Age (y) | 39.85 ± 9.82 | 40.50 ± 9.56 | 43.23 ± 7.64 | 40.60 ± 7.58 | 0.527 |
| Sex | 3 (15.00) | 5 (25.00) | 4 (20.00) | 6 (30.00) | 0.702 |
| Male | |||||
| Female | 17 (85.00) | 15 (75.00) | 16 (80.00) | 14 (70.00) | |
| Duration of surgery (min) | 113.90 ± 26.13 | 122.10 ± 24.08 | 110.29 ± 26.86 | 123.67 ± 22.01 | 0.247 |
| BMI (kg/m2) | 44.73 ± 3.79 | 43.73 ± 3.86 | 44.21 ± 4.27 | 45.37 ± 3.69 | 0.548 |
Abbreviations: TAP, transversus abdominis plane; RM, recruitment maneuver; SD, standard deviation; BMI, body mass index.
Forced vital capacity and FEV1 before the operation were insignificantly different among the four groups. After the operation, both FVC and FEV1 had increased significantly in group IV compared to other groups (P value < 0.001) and were insignificantly different between groups I, II, and III. FEV1/FVC was insignificantly different among the four groups before and after the operation (Table 2).
| Varaibles | Before Operation | After Operation |
|---|---|---|
| FVC (L) | ||
| Group I (Control) (n = 20) | 3.44 ± 0.64 | 2.34 ± 0.61 |
| Group II (TAP) (n = 20) | 3.38 ± 0.47 | 2.50 ± 0.47 |
| Group III (RM) (n = 20) | 3.53 ± 0.53 | 2.56 ± 0.50 |
| Group IV (TAP + RM) (n = 20) | 3.57 ± 0.61 | 3.03 ± 0.57 |
| P value | 0.681 | 0.001 a |
| P1 | - | 0.777 |
| P2 | - | 0.527 |
| P3 | - | < 0.001 a |
| P4 | - | 0.977 |
| P5 | - | 0.010 a |
| P6 | - | 0.030 a |
| FEV1 (L) | ||
| Group I (control) (n = 20) | 3.12 ± 0.61 | 2.03 ± 0.56 |
| Group II (TAP) (n = 20) | 3.03 ± 0.45 | 2.23 ± 0.46 |
| Group III (RM) (n = 20) | 3.18 ± 0.55 | 2.28 ± 0.49 |
| Group IV (TAP + RM) (n = 20) | 3.20 ± 0.62 | 2.77 ± 0.56 |
| P value | 0.754 | < 0.001 a |
| P1 | - | 0.583 |
| P2 | - | 0.390 |
| P3 | - | < 0.001 a |
| P4 | - | 0.989 |
| P5 | - | 0.006 a |
| P6 | - | 0.016 a |
| FEV1/FVC (%) | ||
| Group I (control) (n = 20) | 90.81 ± 6.85 | 86.43 ± 6.03 |
| Group II (TAP) (n = 20) | 89.81 ± 6.15 | 89.29 ± 6.24 |
| Group III (RM) (n = 20) | 90.10 ± 6.06 | 88.90 ± 7.29 |
| Group IV (TAP + RM) (n = 20) | 89.43 ± 5.72 | 91.76 ± 9.73 |
| P value | 0.906 | 0.155 |
Abbreviations: TAP, transversus abdominis plane; RM, recruitment maneuver; FVC, forced vital capacity; FEV, forced expiratory volume; P1, P value between group I and group II; P2, P value between group I and group; P3, P value between group I and group IV; P4, P value between group II and group III; P5, P value between group II and group IV; P6, P value between group III and group IV.
a Significant as P value ≤ 0.05.
Arterial oxygen pressure before and after the operation was insignificantly different among the four groups. Intraoperative PaO2 was significantly higher in groups III and IV compared to other groups (P value < 0.001) and was insignificantly different between groups I and II and between groups III and IV. PaO2/FiO2 before the operation was insignificantly different among the four groups. Intraoperative PaO2/FiO2 was significantly higher in groups III and IV compared to other groups (P value < 0.001) and was insignificantly different between groups I and II and between groups III and IV. PaO2/FiO2 after the operation was insignificantly different among all groups (P value = 0.086) (Table 3).
| Variables | Before Operation | Intraoperative | After Operation |
|---|---|---|---|
| PaO2 (mmHg) | |||
| Group I (control) (n = 20) | 90.00 ± 2.18 | 277.65 ± 27.85 | 88.15 ± 2.23 |
| Group II (TAP) (n = 20) | 90.00 ± 4.08 | 272.95 ± 29.88 | 86.80 ± 4.05 |
| Group III (RM) (n = 20) | 88.70 ± 3.92 | 327.00 ± 22.92 | 85.95 ± 3.78 |
| Group IV (TAP + RM) (n = 20) | 90.25 ± 3.57 | 329.20 ± 15.50 | 88.85 ± 4.22 |
| P value | 0.076 | < 0.001 a | 0.086 |
| P1 | - | 0.687 | - |
| P2 | - | < 0.001 a | - |
| P3 | - | < 0.001 a | - |
| P4 | - | < 0.001 a | - |
| P5 | - | < 0.001 a | - |
| P6 | - | 0.795 | - |
| PaO2/FiO2 | |||
| Group I (control) (n = 20) | 428.60 ± 10.42 | 348.57 ± 34.39 | 419.95 ± 10.51 |
| Group II (TAP) (n = 20) | 428.65 ± 19.28 | 341.38 ± 36.38 | 413.35 ± 19.23 |
| Group III (RM) (n = 20) | 422.45 ± 18.49 | 408.75 ± 28.86 | 412.15 ± 18.76 |
| Group IV (TAP + RM) (n = 20) | 429.80 ± 16.82 | 411.00 ± 20.02 | 423.10 ± 20.09 |
| P value | 0.074 | < 0.001 a | < 0.001 a |
| P1 | - | 0.742 | 0.663 |
| P2 | - | < 0.001 a | 0.119 |
| P3 | - | < 0.001 a | < 0.001 a |
| P4 | - | < 0.001 a | 0.549 |
| P5 | - | < 0.001 a | < 0.001 a |
| P6 | - | 0.847 | < 0.001 a |
Abbreviations: TAP, transversus abdominis plane; RM, recruitment maneuver; FVC, forced vital capacity; FEV, forced expiratory volume; PaO2, partial pressure of oxygen; P1, P value between group I and group II; P2, P value between group I and group III; P3, P value between group I and group IV; P4, P value between group II and group III; P5, P value between group II and group IV; P6, P value between group III and group IV.
a Significant as P value ≤ 0.05.
Heart rate was insignificantly different among the four groups at all times of the measurements (Figure 2).
Mean arterial blood pressure was insignificantly different among the four groups at all times of the measurements (Figure 3).
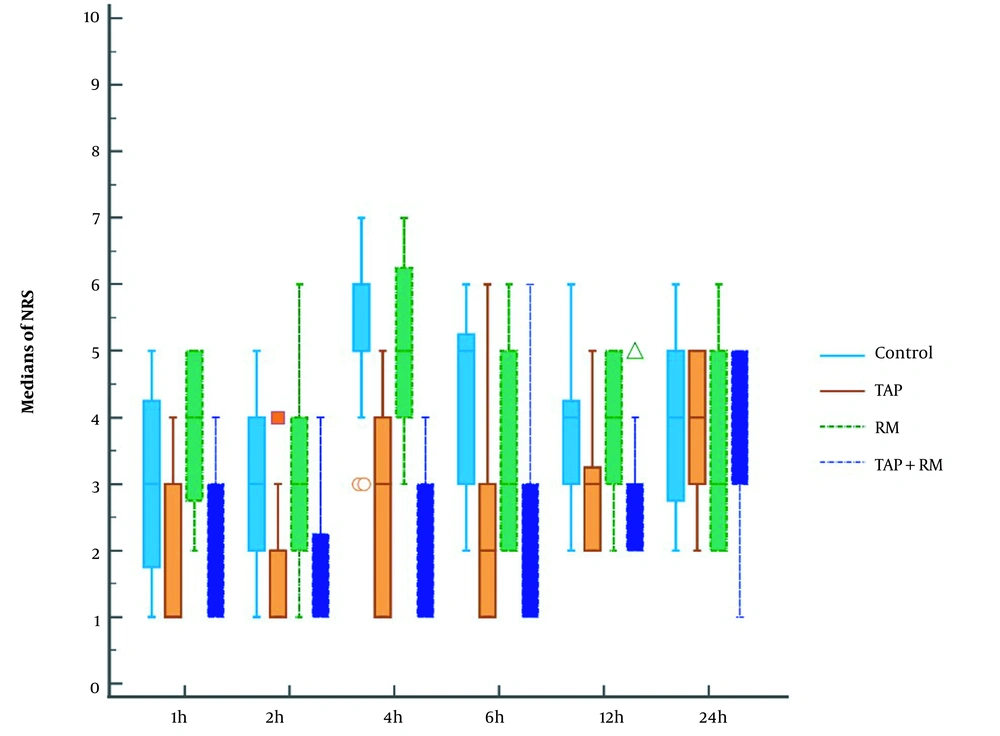
Numerical rating scale at 1, 2, 4, 6, and 12 h decreased significantly in groups II and IV compared to other groups (P value < 0.05) and was insignificantly different between the I and III groups and between II and IV groups. NRS at 24h was insignificantly different among the four groups (Figure 4).
Opioid consumption was significantly lower in groups II and IV compared to other groups and was insignificantly different between groups II and IV and groups I and III (Table 4).
Abbreviations: TAP, transversus abdominis plane; RM, recruitment maneuver; SD, standard deviation; BMI, body mass index; P1, P value between group I and group II; P2, P value between group I and group III; P3, P value between group I and group IV.
a Significant as P value ≤ 0.05.
5. Discussion
Obesity is an epidemic problem with an increasing prevalence rate (15). Obesity is one of the most dangerous chronic diseases (16). There are several types of treatment for patients with severe obesity, for example, pharmacological and behavioral. Bariatric surgery is considered when all other treatments have failed, and it is the most effective treatment option in terms of achieved weight reduction (17).
Despite the great success of bariatric surgery for weight reduction yet, many complications can be encountered because of the challenges that obese patients face with ventilation and airway management in addition to the high incidence of chronic comorbidities that obese patients face, which, augmented by the effect of anesthesia, surgery, positioning and peritoneal insufflation used with bariatric procedures.
Our results showed that intraoperative PaO2 and PaO2/FiO2 were significantly higher in groups III and IV compared to other groups.
Different intraoperative ventilatory techniques are suggested to overcome the adverse response to anesthesia and surgery in the morbidly obese, especially during laparoscopic procedures involving peritoneal insufflation. The use of RM was associated with increased arterial oxygenation due to reopening collapsed alveolar units and increasing lung area available for gas exchange.
In agreement with our results, Chalhoub et al. (18) reported in their study on morbidly obese patients scheduled for bariatric surgery that the addition of PEEP and lung recruitment improves postoperative oxygenation.
In addition, Whalen et al. (19) conducted a randomized study to determine the effect of a lung recruitment maneuver on PaO2 and PaO2/FiO2 after laparoscopic bariatric surgery. They showed that alveolar recruitment successfully enhanced intraoperative oxygenation and briefly elevated the dynamic compliance of the respiratory system.
Also, Costa Souza et al. (20), in a systematic review and meta-analysis, showed that intraoperative alveolar recruitment maneuvers, in addition to the use of PEEP for obese patients, improve gas exchange with increased respiratory system compliance.
Contrary to our results, Defresne et al. (21) reported that lung recruitment did not improve lung function or oxygenation in morbidly obese patients undergoing laparoscopic surgery. Different ventilation strategies can explain this discrepancy with our results as they use low tidal volume and high PEEP in all patients.
Our results showed that morphine consumption and the NRS at 1, 2, 4, 6, and 12h were significantly decreased in groups II and IV compared to other groups.
Many studies have been conducted to evaluate the efficacy of different postoperative pain management techniques in bariatric surgeries to allow early ambulation, decrease opioid consumption, and reduce the incidence of postoperative ventilatory complications.
In agreement with our results, Földi et al. (22) conducted a meta-analysis and systematic review evaluating the effectiveness of perioperative ultrasound-guided TAP block in laparoscopic bariatric surgery as a part of the multimodal analgesic strategy. Which demonstrated decreased pain scores and opioid use with TAP block.
In the same line, Chaw et al. (23) conducted a comprehensive review and meta-analysis of randomized controlled trials to assess the analgesic effectiveness of TAP block, which demonstrated that TAP block is superior in lowering opioid use, improving pain scores, and minimizing postoperative nausea and vomiting.
In contrast to our results, Tülübaş et al. (24) designed a prospective randomized study in patients who underwent laparoscopic sleeve gastrectomy. The results showed that there was not any significant difference in both groups regarding postoperative opioid consumption. However, the pain score was significantly higher in the control group. This can be justified by the different approaches to TAP block and the difference in the analgesic regimen used in the control group.
Also, Albrecht et al. (13) designed a prospective randomized controlled trial in patients who underwent laparoscopic gastric bypass surgery. The results showed that there was no significant difference in cumulative opioid consumption and pain severity score with TAB block. This can be justified by trocar insertion site local anesthetics infiltration and different systemic analgesia.
To our knowledge, the present study was the first one that mentioned the effect of intraoperative lung recruitment combined with the analgesic effect of TAP block in laparoscopic bariatric surgery on postoperative lung functions.
Our results showed that both FVC and FEV1 were found to be significantly higher after operation in group IV compared to other groups. FEV1/FVC was insignificantly different among the four groups before and after the operation.
Limitations of the study were a relatively small sample size, a shorter duration of follow-up, and the fixed PEEP used. Further studies with larger sample sizes, comparing different recruitment maneuvers with different PEEP levels and longer duration of postoperative follow-up, are needed to verify these results. RM groups showed higher intraoperative PaO2 and PaO2/FiO2. TAP groups showed lower NRS and opioid consumption in the postoperative period. In addition, the combination of RM and TAP showed higher postoperative FVC and FEV1.