1. Background
Magnetic resonance imaging (MRI) is progressively being utilized in different conditions in pediatrics due to its excellent soft-tissue contrast resolution and ability to assess the structure and function of body organs (1). Although MRI has benefits over other imaging modalities, it has several drawbacks, such as long acquisition periods, closed space, and loud noises (2). Moreover, MRI is affected by patient movement, which can lower the image quality and increase the risk of misdiagnosis (3, 4).
The use of general anesthesia (GA) has become the standard choice in MRI for children with neurological impairments, global developmental delay, significant behavioral disorders, or who cannot otherwise comply with the instructions required to provide suitable MRI images (5). GA is used to obtain better MRI image quality, which is considered challenging in pediatrics (1). Radiologists must actively perform quality control for sedated/anesthetized patients and maintain continual communication with MRI technologists (6).
Airway management during GA involves several techniques, including oral airway, endotracheal tube, and numerous supraglottic airway (SGA) devices. Laryngeal mask airway (LMA), as one of these devices, has been used to prevent airway obstruction and enhance the MRI image quality (7). In previous research on serial MRIs performed on anesthetized children and adults, SGA was shown to minimize motion artifacts and improve image quality significantly (3). The LMA has been widely used in pediatric anesthesia in various surgical procedures. It provides significant advantages over endotracheal tubes because of its supraglottic position, which reduces the occurrence of laryngospasm, cough, and postoperative desaturation (8-10).
2. Objectives
We hypothesized that LMA would improve brain MRI image quality compared to Guedel airway. Therefore, we aimed to compare LMA versus oral airway for airway management during brain MRI in terms of reducing motion artifacts and providing better image quality.
3. Methods
This randomized, controlled, double-blind trial was performed on 40 pediatrics aged 1 - 18 years, from both genders, and American Society of Anesthesiology (ASA) І and П scheduled for brain MRI at the National Cancer Institute, Cairo University, Egypt, from February 2021 to March 2022. The study was conducted after approval from the Ethics Committee (AP2007-50111) and registration at clinicaltrials.gov (ID: NCT04730362). Informed written consent from the patient or their guardians was obtained. Exclusion criteria were emergency patients with a full stomach, Glasgow Coma Scale (GCS) ≤ 8, and anatomical abnormalities of the upper airway necessitating endotracheal intubation.
3.1. Randomization and Blindness
A statistician unrelated to patient treatment used a computer-generated program (permuted block technique) to randomize the patients in a parallel manner into two equal groups (group ratio 1: 1) based on the method of airway management. In the control group, Guedel oral airway was used, and in the LMA group, LMA was applied. In both groups, participants and radiologists were blinded. All patients underwent complete history taking, clinical examination, especially for airway, and routine laboratory investigations. An MRI-compatible monitor was used to measure mean arterial blood pressure (MAP), heart rate (HR), and peripheral oxygen saturation (SpO2). A Capnogram and visual inspection of the chest were used to assess adequate ventilation. The pulse oximetry and capnography used in the study were compatible with MRI.
After intravenous (IV) line placement and securing, 0.01 mg/kg atropine was administered as an anti-sialagogue to reduce oral secretion. Anesthesia was induced by 2 mg/kg propofol IV boluses and maintained with O2 and sevoflurane 2% - 4% by a compatible anesthesia machine. The LMA and airway were inserted after diminishing eyelid/blink reflex or/and after apnea onset. A bolus of 1 mg propofol was administered as rescue medication if the patient did not tolerate an LMA or airway. If the patient developed apnea, ventilation was assisted till regaining spontaneous breathing. The mean sevoflurane percent was recorded. Patients were spontaneously ventilated through LMA or airway according to the group. MAP, HR, and SpO2 were recorded at baseline, immediately after oral airway/LMA insertion, 10, 20, and 30 min after insertion, pre-extubation, immediately after oral airway/LMA removal, as well as 5 and 10 min post-removal.
3.2. Guedel Oral Airway Group
Using anatomical landmarks, the optimal oropharyngeal size was determined individually. Externally, the flange should approach the lips, and the tip should be able to reach the angle of the jaw. A harness fixed the mask over the airway.
3.3. Laryngeal Mask Group
The LMA (Classic®) was used for airway management. The size of LMA was chosen according to the weight of the patients and the volume of the inflated air in the seal ring according to the manufacturer's recommendation. The LMA was positioned through the conventional technique, partially inflated, and the tip of the mask was lubricated by clear liquid lubricating jelly after the induction of anesthesia with the patient’s head in the neutral position. The LMA proper position was described as placement in the hypopharynx, the proximal cuff of LMA opposing C1 or C2 vertebrae, with a distance between proximal cuff end and aditus laryngis (distance A) as well as between distal cuff end and aditus laryngis (distance B) (11). If LMA or airway displacement during anesthesia occurred, their position was corrected, and MRI was restarted.
3.4. MRI Image Quality Assessment
A 1.5 or 3 T system was utilized to perform MRI. Six conventional MRI sequences, including axial T2, axial fluid-attenuated inversion recovery (FLAIR), axial T1 pre-contrast, and T1 post-contrast in the axial, coronal, and sagittal planes, were examined. Scores were given by a neuroradiologist with more than 20 years of experience. A scoring system assessed the image quality of each MRI sequence with the following scores: 1 (non-diagnostic), 2 (poor quality but with some diagnostic utility), 3 (average), 4 (good), and 5 (excellent). A total score ranging from 6 to 30 was assigned to each MRI session. Any airway complications, such as cough, sore throat, laryngeal spasm, bronchospasm, and anesthetic complications (nausea and vomiting), were recorded from induction until 1 h post-procedure. The primary outcome was the MRI image quality score; the secondary outcomes were airway and anesthetic complications.
3.5. Sample Size
The required sample size was obtained by G. power (Universitat Kiel, Germany) 3.1.9. 2. It was based on an α error of 0.05, 90% power, and the mean ± SD of MRI image quality score of 27.6 ± 2.3 with SGA and 20.3 ± 4.7 with oral airway, according to a previous study (3). Six pediatrics were added to each group to overcome dropout during the follow-up. Therefore, 20 patients were allocated to each group.
3.6. Statistical Analysis
IBM SPSS version 26 (Chicago, Illinois, United States) was utilized for statistical analysis. The normality of data distribution was assessed using the Shapiro-Wilk test and histograms. Quantitative parametric data were presented as mean ± SD and were analyzed by the unpaired student t-test. Quantitative non-parametric data were presented as the median and interquartile range (IQR) and analyzed using the Mann-Whitney test. Repeated measures analysis of variance (ANOVA) was used to compare the serial measurements of MAP, HR, and SpO2. Qualitative variables were presented as frequency and percentage (%) and were analyzed using the chi-square test. A two-tailed P-value ≤ 0.05 was considered statistically significant.
4. Results
In this trial, 64 pediatrics were evaluated for eligibility, 21 patients did not meet the inclusion criteria, and three patients’ guardians refused to participate in the trial. The remaining 40 patients were randomly allocated into two equal groups. All enrolled cases were followed-up and analyzed (Figure 1).
Patients’ demographic data, including age, weight, height, body mass index (BMI), gender, and ASA physical status, were insignificantly different between the two groups (Table 1).
| Variables | Control Group (n = 20) | LMA Group (n = 20) | P-Value | Mean Difference/RR (95% CI) |
|---|---|---|---|---|
| Age (y) | 8.50 ± 4.87 | 7.40 ± 4.90 | 0.481 | 1.1 (-2.029: 4.229) |
| Weight (kg) | 33.4 ± 17.99 | 30.95 ± 18.19 | 0.671 | 2.45 (-9.132: 14.032) |
| Height (m2) | 1.24 ± 0.26 | 1.19 ± 0.25 | 0.559 | 0.05 (-0.115: 0.209) |
| BMI (kg/m2) | 21.85 ± 273.09 | 21.88 ± 295.9 | 0.885 | -0.03 (-2.677: 2.618) |
| Sex | 0.749 | 1.44 (0.808: 2.583) | ||
| Male | 13 (60.0) | 9 (45.0) | ||
| Female | 7 (40.0) | 11 (55.0) | ||
| ASA physical status | 0.526 | 1.29 (0.596: 2.774) | ||
| I | 9 (45.0) | 7 (35.0) | ||
| II | 11 (55.0) | 13 (65.0) |
Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index; CI, confidence interval; LMA, laryngeal mask airway; RR, relative risk.
a Values are expressed as mean ± SD or No. (%).
In addition, the duration of MRIs and anesthesia were insignificantly different between the groups. The mean sevoflurane percent and patients who required propofol boluses were comparable between the two study groups (Table 2). The mean ± SD of the total MRI image quality score was 26.10 ± 3.97 in the LMA group versus 18.60 ± 5.30 in the control group. Total MRI image quality scores were significantly higher in the LMA group than in the control group (P < 0.001) (Table 2).
| Variables | Control Group (n = 20) | LMA Group (n = 20) | P-Value | Mean Difference (95% CI) |
|---|---|---|---|---|
| Duration of MRI scan (min) | 42.85 ± 8.8 | 46.55 ± 8.56 | 0.186 | -3.7 (-9.259: 1.859) |
| Duration of anesthesia (min) | 48.05 ± 8.9 | 50.6 ± 9.12 | 0.376 | -2.55 (-8.317: 3.217) |
| Sevoflurane (%) | 3.47 ± 0.3 | 3.54 ± 0.44 | 0.557 | -0.07 (-0.309: 0.169) |
| Patients required propofol boluses | 2 (10) | 4 (20) | 0.422 | 0.5 (0.103: 2.43) |
| Total MRI image quality score | 18.60 ± 5.30 | 26.10 ± 3.97 | < 0.001 | -7.5 (-10.497: -4.503) |
Abbreviations: CI, confidence interval; LMA, laryngeal mask airway; MRI, magnetic resonance imaging.
a Values are expressed as mean ± SD or No. (%).
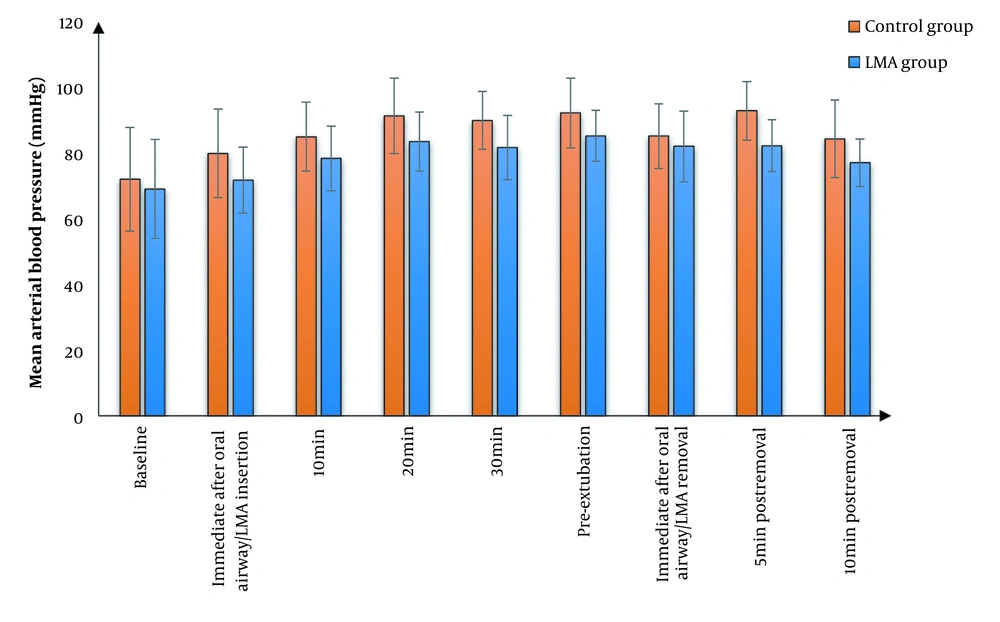
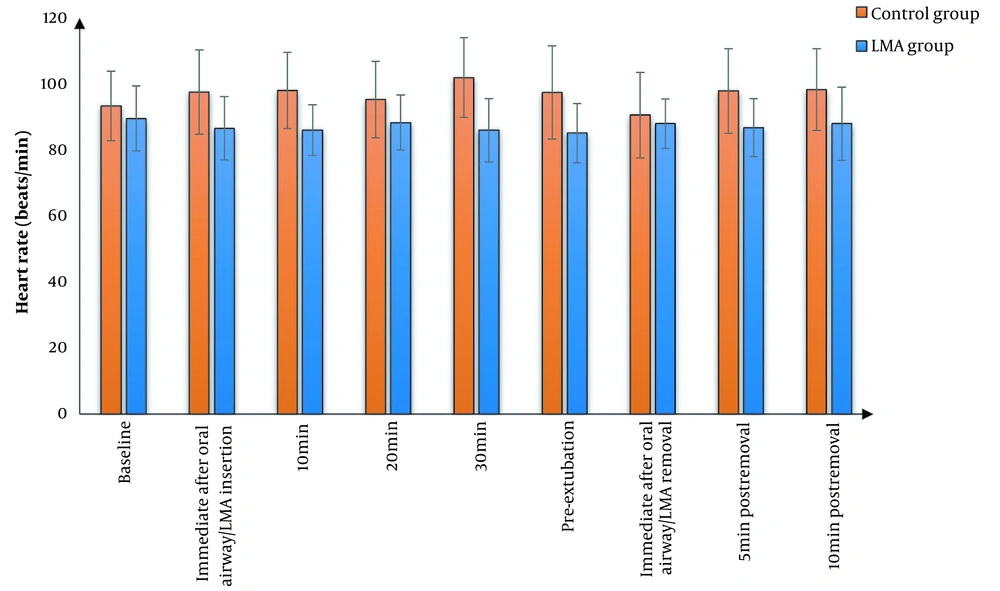
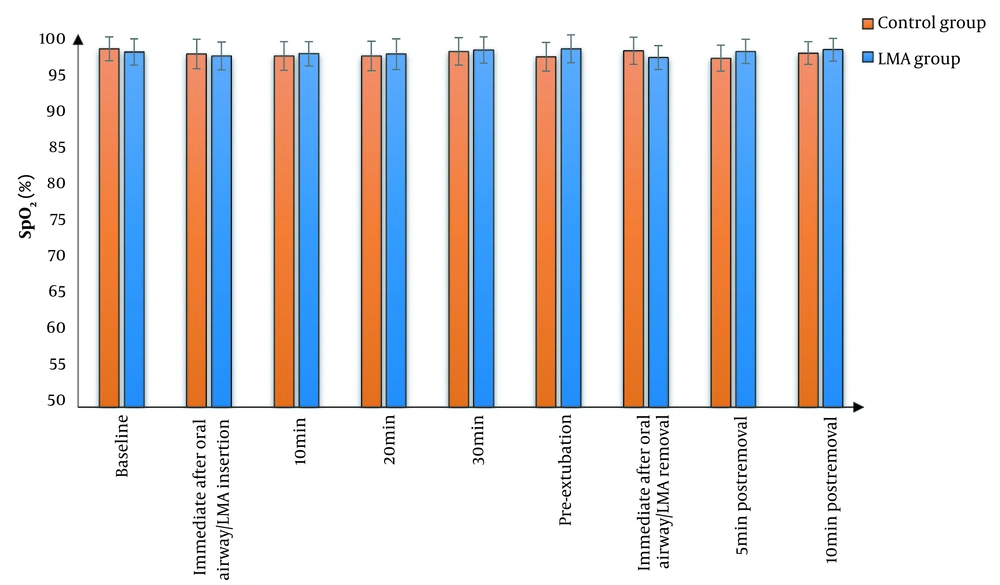
We found that MAP and HR were significantly lower in the LMA group than in the control group at all study times except at baseline and immediate post-extubation, where there was an insignificant difference between the two groups. The effects of time and group factors were significant. Furthermore, the two groups did not have a significant difference in terms of SpO2 (Figures 2 - 4). Cough was significantly lower in the LMA group than in the control group (15% versus 50%, P = 0.040). Airway complications, namely sore throat, laryngeal spasm, and bronchospasm, as well as anesthesia complications (e.g., nausea and vomiting), were insignificantly different between the study groups (Table 3).
| Variables | Control Group (n = 20) | LMA Group (n = 20) | P-Value | RR (95% CI) |
|---|---|---|---|---|
| Cough | 10 (50.0) | 3 (15.0) | 0.040 | 3.33 (1.075: 10.335) |
| Sore throat | 7 (35.0) | 3 (15.0) | 0.273 | 2.33 (0.701: 7.764) |
| Laryngeal spasm | 4 (20.0) | 2 (10.0) | 0.661 | 2 (0.412: 9.712) |
| Bronchospasm | 5 (25.0) | 2 (10.0) | 0.407 | 2.5 (0.548: 11.41) |
| Nausea and vomiting | 7 (35.0) | 3 (15.0) | 0.273 | 2.33 (0.701: 7.764) |
Abbreviations: CI, confidence interval; LMA, laryngeal mask airway; RR, relative risk.
a Values are expressed as No. (%).
5. Discussion
Airway management during MRI is a critical concern for anesthesiologists (12). Different variables can impact the image quality of MRI scans. Movement of the patient during the operation may interfere with image interpretation and disrupt the scan, which may necessitate repeating the procedure. Therefore, GA produces better MRI image quality than sedation because of preventing patient movement (13).
The LMA has also gained more popularity in MRI in pediatrics in the past years through airway complication rate reduction, lower intrusive nature, excellent clinical performance, and low failure rate (11). Our results presented that the MRI scan performed by LMA was superior to Guedel oral airway in image quality with comparable needed anesthesia (the mean sevoflurane percent and patients who required propofol boluses were comparable between both groups). In agreement with our result, Wu et al. (12) found that MRI scans were completed without interruption with adequate image quality with SADs and concluded that LMA is an acceptable airway alternative for pediatric MRI scans of the central nervous system when the pilot balloon is carefully placed outside the MRI field.
Moreover, Zaballos et al. (14) conducted an in vitro simulation study to investigate the artifacts created during MRI by six distinct types of SADs, including the classic LMA and other types of SADs. They reported that no artifacts occurred in the MRI scans. Ucisik-Keser et al. (3) recently observed that MRI scans performed using SGAs had better brain MRI image quality in pediatric and adult patients among four airway management strategies. The arithmetic means for image quality scores were 27.6 with SGA and 20.3 with the oral airway. The minimal scores also differed significantly between the SGA and oral airway groups, indicating that the application of SGA guaranteed consistently good MRI image quality.
In our study, MAP was significantly lower in the LMA group than in the control group at most measurement periods. In line with our results, Jain et al. (15) showed that the placement of LMA before tracheal extubation is associated with reduced hemodynamic changes (minimal changes in blood pressure and HR) in comparison with Guedel oropharyngeal airway for tracheal tube exchange and smooth extubation. Moreover, Taheri et al. (16) demonstrated that LMA offered several advantages compared to alternative methods of airway management in reducing the risk of vocal cord paralysis and minimal cardiovascular reaction, with only 12.3% of patients in the trial suffering from hemodynamic instability during major ear surgery.
In the present study, cough significantly decreased with LMA compared to controls (15% vs. 50%, P = 0.040). Airway complications were insignificantly different between the two groups. This was in line with Wu et al. (12), who showed that using reusable LMA and i-gel for airway preservation during MRI is practical and safe for pediatrics under MRI scans with minimal complications. Taheri et al. (16) found observed cough in only 10 (0.9%) children following surgery, most commonly in the age ranges of 3 - 7 and 8 - 15 years; however, sore throat was observed in only two (0.01%) patients who had used LMA in major ear surgery.
One of the limitations of using LMA is its higher cost compared to the oral airway. Furthermore, LMA cannot be used in certain circumstances, such as GCS ≤ 8 and emergency patients with a full stomach. Limitations of our study included a small sample size to prove secondary outcomes and a wide range of ages. In addition, MRI-compatible bispectral index or other monitors for the depth of anesthesia were not available in our trial. Further studies are needed on a larger scale to assess other SGA types and endotracheal tubes.
Compared to the Guedel oral airway, using LMA for airway management in pediatrics undergoing MRI scans improved the image quality with less cough and better hemodynamics.