1. Background
Strabismus surgery is one of the most common ophthalmic surgeries to align the 2 eyes. This surgery is performed in different ways (1). One of the most common and important complications of this surgery is pain and a high rate of postoperative nausea and vomiting (PONV) (2). Postoperative nausea and vomiting is the most common complication after strabismus surgery under general anesthesia, with an incidence of 37 - 80% (3). The severity and frequency of PONV after surgery depend on various factors, such as the type of surgery, the amount of opioid use during and after surgery, anesthesia, and patient-related factors, such as female gender, PONV history, smoking, etc. (4, 5). Postoperative nausea and vomiting can be associated with an increase in the duration of hospitalization and complications such as dehydration, water and electrolyte disorders, impaired healing of the surgical wound, exacerbation of pain, and decreased patient satisfaction.
Nausea and vomiting have a complex mechanism in which many neurotransmitters are involved. In this regard, although there is no single drug that can completely prevent this complication (6), there are various pharmacological and non-pharmacological methods to control and reduce this complication. Among the drugs used are traditional antiemetic drugs, such as metoclopramide and dimenhydrinate, non-traditional antiemetic drugs, such as dexamethasone, midazolam, clonidine, and lidocaine, and anti-serotonin drugs, such as ondansetron, which have been used in various studies and each has been effective to some extent (7, 8). One of the non-traditional drugs is dexamethasone, which has analgesic, anti-inflammatory, immunomodulatory, and antiemetic effects (9). Its possible antiemetic mechanism is the inhibition of prostaglandins, inhibition of serotonin release in the intestine, reduction of 5-hydroxytryptophan nerve levels, and release of endorphins (10).
In recent years, intranasal administration of drugs has gotten increasingly noticed due to benefits such as non-invasiveness, the possibility of use by the patient himself, faster onset of action, and higher bioavailability (due to bypass of hepatic first-pass metabolism) (11). Many drugs can be prescribed intranasally, including benzodiazepines (such as midazolam) (11), fentanyl (12), and corticosteroids (such as dexamethasone) (13). Nowadays, the use of nasal dexamethasone is limited to cases such as allergic rhinitis, rhinosinusitis, and nasal polyps (14, 15).
2. Objectives
Despite many studies around the world on the antiemetic effects of intravenous dexamethasone, the effect of intranasal administration has not been studied. On the other hand, due to the speed and ease of use of intranasal drugs, the present study was performed to investigate the preventive effect of intranasal dexamethasone on the incidence of nausea and vomiting after adult strabismus surgery.
3. Methods
This study is a randomized, double-blind controlled clinical trial study that is approved by the code IR.MUI.MED.REC.1398.563 in the Ethics Committee of Isfahan University of Medical Sciences with the ID IRCT20180416039326N12 in the Iranian Clinical Trial Registration Center and was performed in 2020 at Feiz Eye Hospital of Isfahan. Inclusion criteria were class 1 and 2 American Society of Anesthesiologists (ASA), age range of 18 - 65 years, candidate for strabismus surgery, and informed consent to participate in the study. Also, patients with bilateral strabismus, motion sickness, receiving antiemetic drugs in the past 24 hours before surgery, patients treated with opioids, smokers, and pregnant women were not included in the study. Changes in anesthesia schedule and procedure, severe hemodynamic disturbances during surgery, and transferring the patient to the intensive care unit were considered exclusion criteria. The minimum number of cases needed for each group was 30 people based on a similar article (the effect of dexamethasone on prevention of nausea and vomiting after thyroidectomy) (16) considering the first and second type errors of 0.05 and 0.2 and the expected percentage of 28.5 and 71.4% for intervention groups.
The patients were entered into the study using the table of random numbers resulting from the randomization allocation software in 2 groups of 36 people. In this double-blind study, the patient, the surgeon, and the person who collected the data were unaware of the patient’s grouping. The drug and placebo were prepared in similar, coded syringes by an anesthesia nurse who was not a member of the research team and provided to the project manager. Patients were explained about the drugs under study and the visual analog score (VAS) system for assessing nausea and pain. Patients’ demographics, ASA functional class, and baseline vital signs were recorded in a checklist. In the operating room, all patients underwent electrocardiogram monitoring, non-invasive intermittent blood pressure measurement, pulse oximetry, and capnography. The duration of fasting (NPO) and serum therapy of the patients were the same. 5 cc/kg of ringer serum was infused for patients. All patients underwent standard general anesthesia, including pre-oxygenation and induction of anesthesia with fentanyl 2 μg/kg, propofol 2 mg/kg, and atracurium 0.6 mg/kg.
Tracheal intubation of patients was performed with tube number 8 in men and tube number 7.5 in women. Immediately after inflating the cuff, the first group (intranasal dexamethasone (ND)) received dexamethasone (Dexamethasone DP 8 mg/2 mL Amp, Darou Pakhsh Holding Company), 1 mL in each nasal passage, and the second group (intranasal normal saline (NS)) received 1 mL of normal saline per nasal passage. Maintenance of anesthesia continued with an infusion of propofol 3 - 6 mg/kg/h, and all patients received 0.1 mg/kg morphine during surgery. After surgery, the remaining neuromuscular block was reversed by 0.04 mg/kg neostigmine and 0.02 mg/kg atropine, and after vigilance and effective respiratory recovery, patients were extubated. The time of extubation (from the point of discontinuation of the anesthetic to the point of extubation) was recorded. The length of stay in recovery was assessed and recorded by the modified Aldrete score system (17).
The severity of postoperative nausea and vomiting was scored on a scale ranging from 0 to 3. In this criterion: Score 0: No nausea and no vomiting; score 1: Presence of nausea but no vomiting; score 2: Presence of nausea and vomiting; and score 3: Vomiting occurs more than twice every 30 minutes. The severity of postoperative nausea and pain was also assessed using VAS and recorded in the first 0 - 2 hours and 2 - 24 hours after surgery (18). Patients with nausea and vomiting with a score of 2 or more received 4 mg of ondansetron intravenously and patients with pain with a VAS score of more than 4 received 25 mg of pethidine, and its dose was recorded. Patients’ satisfaction was scored on a Likert scale from 1 - 5 (including completely dissatisfied, dissatisfied, neutral, satisfied, and completely satisfied, respectively). Finally, data were entered into the SPSS version 23 software (IBM SPSS, Armonk, NY, USA) and analyzed by chi-square, t-test, paired t-test, Mann-Whitney, and repeated measures analysis of variance (ANOVA). In the study, a P-value < 0.05 was considered statistically significant.
4. Results
In this study, 72 patients who were candidates for strabismus surgery were divided into 2 groups of 36, receiving ND or NS. No patients were excluded during the study due to adverse events or other exclusion criteria. According to Table 1, the 2 groups did not differ significantly in terms of distribution of demographic and baseline variables.
| Variables | Groups | P-Value | |
|---|---|---|---|
| Intranasal Dexamethasone | Intranasal Normal Saline | ||
| Age (y) | 29.39 ± 9.81 | 27.28 ± 7.29 | 0.304 |
| Gender | 0.81 | ||
| M | 16 (44.4) | 17 (47.2) | |
| F | 20 (55.6) | ||
| Weight (kg) | 69.92 ± 23.35 | 64.31 ± 12.45 | 0.21 |
| ASA | 1 | ||
| 1 | 31 (86.1) | 31 (86.1) | |
| 2 | 5 (13.9) | 5 (13.9) | |
Distribution of Demographic and Baseline Variables Between the 2 Groups a
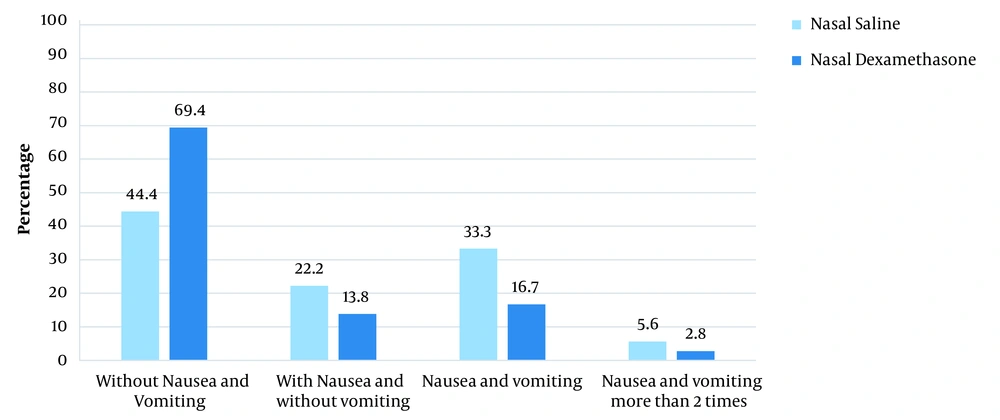
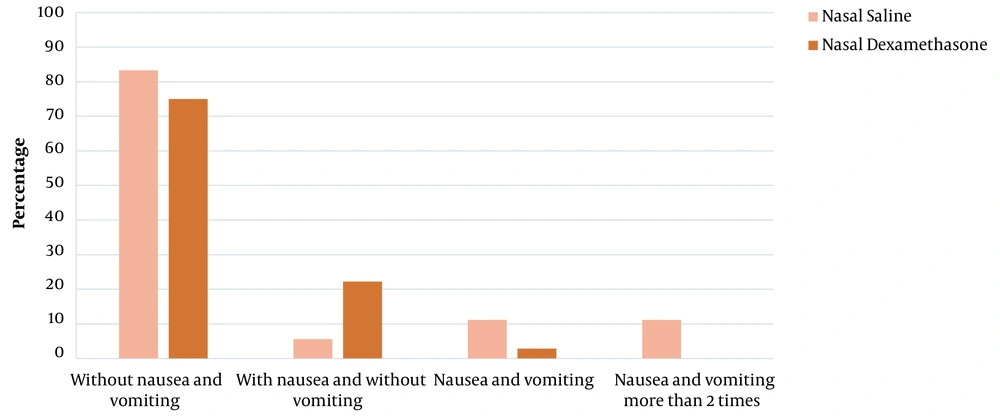
There was no significant difference between the 2 groups in terms of mean age (P = 0.304), weight (P = 0.21), gender frequency (P = 0.81) and ASA (P = 1). According to the results, 2 hours after surgery, the incidence of nausea (P = 0.032) and its severity (P = 0.019) were significantly lower in the ND group than in the NS group. In 2 - 24 hours after the surgery, there was no significant difference between the 2 groups in terms of nausea (P = 0.38) and its severity (P = 0.55). The frequency of vomiting in 2 hours (P = 0.13) and 2 - 24 hours after the surgery (P = 0.08) did not differ between the 2 groups (Table 2 and Figure 1).
| Variables | 2 Hours Postoperative | 2 - 24 Hours Postoperative | ||||
|---|---|---|---|---|---|---|
| Intranasal Dexamethasone | Intranasal Normal Saline | P-Value | Intranasal Dexamethasone | Intranasal Normal Saline | P-Value | |
| Incidence of nausea | 11 (30.6) | 20 (55.6) | 0.032 | 9 (25) | 6 (16.7) | 0.38 |
| Severity of nausea | 2.96 ± 0.49 | 3.42 ± 0.57 | 0.019 | 0.97 ± 0.16 | 0.61 ± 0.28 | 0.55 |
| Incidence of vomiting | 6 (16.7) | 12 (33.3) | 0.11 | 1 (2.8) | 4 (11.1) | 0.16 |
| Frequency of vomiting | 0.13 | 0.08 | ||||
| 0 | 30 (83.3) | 24 (66.7) | 35 (97.2) | 32 (88.9) | ||
| 1 | 1 (2.8) | 7 (19.4) | 0 (0) | 0 (0) | ||
| 2 | 3 (8.3) | 4 (11.1) | 1 (2.8) | 0 (0) | ||
| 3 or more | 2 (5.6) | 1 (2.8) | 0 (0) | 4 (11.1) | ||
Distribution of Severity and Frequency of Postoperative Nausea and Vomiting in the First 2 Hours and 2 - 24 Hours After the Surgery a
Two hours after the surgery, 32 patients (88.9%) in the NS group and 19 patients (52.8%) in the ND group had pain, and the frequency of pain was significantly higher in the NS group than in the ND group (P = 0.001). In 24 hours after the surgery, 22 patients (61.1%) from the NS group and 7 patients (19.4) from the ND group had pain and the difference was significant between the 2 groups (P < 0.001). The mean pain intensity was also significantly higher in the NS group at 2 and 24 hours after the surgery (P < 0.001) (Figure 1).
The mean tolerance time of liquids and solids was not significantly different between the 2 groups. The mean extubation time in the NS group was significantly higher (P < 0.001). Also, the length of recovery time was significantly longer in the NS group. To control nausea and vomiting, 23 patients received ondansetron, 16 of whom were from the NS group and 7 from the ND group, and the difference was significant between the 2 groups (P = 0.023). Pethidine intake was also significantly higher in the NS group (P < 0.001). In terms of patient satisfaction, the ND group had a higher level of satisfaction (P = 0.031). The results are shown in Table 3. It should be noted that except for nausea, vomiting, and pain, 4 patients in the NS group had a sore throat (P = 0.12) and no other complication was observed in the patients.
| Variables | Groups | P-Value | |
|---|---|---|---|
| Intranasal Dexamethasone | Intranasal Normal Saline | ||
| Incidence of pain | |||
| 2 hours after surgery | 19 (52.8) | 32 (88.9) | 0.001 |
| 2 - 24 hours after surgery | 7 (19.4) | 22 (61.1) | < 0.001 |
| Pain intensity | |||
| 2 hours after surgery | 1.94 ± 0.38 | 5.03 ± 3.03 | < 0.001 |
| 2 - 24 hours after surgery | 0.5 ± 0.18 | 2.64 ± 0.44 | < 0.001 |
| Fluid tolerance time (min) | 149 ± 19.7 | 126.7 ± 76.9 | 0.35 |
| Solid foods tolerance time (min) | 256.8 ± 141.8 | 218.6 ± 122.5 | 0.23 |
| Extubation time (min) | 20.47 ± 10.41 | 25.75 ± 7.6 | < 0.001 |
| Recovery time (min) | 72.2 ± 18.4 | 83.5 ± 24.1 | 0.03 |
| Receiving ondansetron | 7 (19.4) | 16 (44.4) | 0.023 |
| Receiving pethidine | 4 (11.1) | 15 (41.6) | < 0.001 |
| Postoperative sore throat | 0 (0) | 4 (11.1) | 0.12 |
| Patient’s satisfaction | 0.031 | ||
| Completely satisfied | 22 (61.1) | 10 (27.8) | |
| Satisfied | 6 (16.7) | 9 (25) | |
| Neutral | 5 (13.9) | 5 (13.9) | |
| Dissatisfied | 2 (5.6) | 6 (16.7) | |
| Completely dissatisfied | 1 (2.8) | 6 (16.7) | |
Incidence and Postoperative Pain Intensity, Time to Tolerate Liquids and Solids, Mean Extubation and Recovery Time, Receiving Antiemetic and Analgesic, and Satisfaction Between the 2 Groups a
5. Discussion
Postoperative nausea and vomiting are common side effects after surgery, for which no unified theory has been proposed so far. This study was conducted to determine the effect of intranasal dexamethasone on reducing the incidence of nausea and vomiting after strabismus surgery in adults. The findings of the study did not show a significant difference between the 2 groups of ND and NS in terms of the distribution of demographic and baseline variables, and no confounding effect of the above factors on pain and PONV intensity was observed. According to the obtained results, 2 hours after the surgery, the occurrence of nausea and its severity were significantly lower in the ND group than in the NS group. In 2 - 24 hours after the surgery, the incidence of nausea and its severity were insignificantly lower in the ND group.
The frequency and severity of vomiting 2 and 24 hours after the operation in the ND group were lower than in the saline group, but the difference was insignificant. The frequency of pain and its average intensity at 2 and 24 hours after the surgery were significantly lower in the ND group. Ondansetron and pethidine intake, extubation time, and recovery time were significantly less in the ND group. Patients’ satisfaction was significantly higher in the ND group. In Liu et al.’s study, it has been shown that dexamethasone can safely reduce PONV caused by various surgeries, including strabismus surgery, thyroidectomy, and tonsillectomy in children (19). This study has shown that dexamethasone was effective in reducing 24-hour PONV, but it was not significantly different from placebo in terms of early PONV.
Ghaheri Najafabadi et al. showed that intravenous injection of 10 mg of dexamethasone in women undergoing thyroidectomy had a significant effect in reducing PONV following surgery in the immediate postoperative period, 4, 8, 12, and 16 hours after surgery compared to placebo (16). Furthermore, it has no special side effects. However, no difference was observed between the 2 groups 20 and 24 hours after the operation (16). In Gandomi et al.’s study, intravenous dexamethasone with a dose of 8 mg was declared effective in reducing the incidence of nausea and vomiting after tympanomastoid surgery (20). In a meta-analysis, De Oliveira et al. reported that a dose of 4 to 5 mg of dexamethasone compared to a dose of 8 to 10 mg seems to have similar clinical effects in reducing PONV (21). In the present study, intranasal dexamethasone was associated with a reduction in PONV, and it is consistent with the studies above. In the study of Rahimi and Foladfar, intravenous injection of 8 mg of dexamethasone was associated with a decrease in the incidence and severity of postoperative pain in inguinal hernia surgery, as well as a decrease in the length of stay in recovery (22). Our study showed that the use of 8 mg of intranasal dexamethasone is associated with a reduction in postoperative pain, a reduction in the need for painkillers, and a reduction in recovery time, which is consistent with the results of the above study (22). According to the findings of the present study, intranasal dexamethasone was effective in reducing the amount of ondansetron intake. Also, intranasal dexamethasone decreased the extubation time compared to the saline group. Patients receiving dexamethasone had a higher level of satisfaction. One of the strengths of the present study is that, for the first time, intranasal dexamethasone has been used to prevent nausea and vomiting after surgery.
Among the limitations of this study, we can mention the small sample size, fixed-dose of dexamethasone, conducting the study in one hospital, and lack of follow-up of other side effects of intranasal dexamethasone, including its effect on blood sugar, especially in diabetic patients. Therefore, conducting multicenter studies with a larger sample size and different doses of intranasal dexamethasone is suggested.
5.1. Conclusions
The findings of the present study show that the intranasal use of dexamethasone with a dose of 8 mg compared to saline is associated with a decrease in PONV and postoperative pain, a decrease in the use of ondansetron and pethidine, and an increase in patient’s satisfaction. Intranasal use of dexamethasone may be an effective and safe method, especially in cases where we do not have access to an intravenous line.