1. Background
Craniosynostosis is defined as premature closure of one or more skull sutures resulting in abnormal skull growth and other adverse effects for the brain beneath. This common disorder occurs approximately in 0.6 per 1,000 births (1). Craniosynostosis, particularly multicultural ones, leads to an intracranial pressure increase, a cerebral growth impairment, and eventually an impaired cognitive performance if left untreated. Therefore, surgical correction of craniosynostosis should be performed within the first year (2, 3). Total calvarial remodeling (TCVR) and frontal orbital advancement remodeling (FOAR) are among the surgical procedures that can be employed to correct craniosynostosis. As compared with invasive procedures, TCVR has been indicated to associate with higher costs, prolonged operative time, higher volumes of blood loss, and longer length of stay (4-6). The most noticeable point is the considerable volume of blood loss due to the manipulation of extensive vessels during this surgical procedure. This substantial blood loss poses a unique blood transfusion requirement, leading to serious short- and long-term complications (7).
In addition, higher volumes of blood loss can be regarded as a major concern for both anesthetists and surgeons. The review of current literature highlights the patients’ noticeable blood loss, which ranges within 20 - 500% of their total circulating volume, during this surgery (5, 8, 9). The mentioned high volume of blood loss leads to higher blood transfusion requirements, which in turn are associated with higher wrong transfusions, allergy, and infection risks. Moreover, the risk of transfusion-associated adverse events is of particular significance in infants < 12 months old (10).
Various strategies have been developed and experimented with in pertinent studies to minimize the blood transfusion requirement. Normovolemic hemodilution (11), intraoperative autologous blood transfusion (12), fibrin sealants (13, 14), injection of epinephrine containing anesthetic to the scalp (15), preoperative autologous blood donation (16), and erythropoietin (17) are among strategies used for reducing the blood transfusion requirement caused by the craniosynostosis corrective surgery.
The use of antifibrinolytic agents such as tranexamic acid (TXA) has recently caught great attention. Therefore, various studies have evaluated the efficacy of this agent. Many studies reported successful outcomes due to reduced perioperative blood loss and also diminished transfusion requirements (18-20). The other approach favored by numerous anesthesiologists is induced hypotension during surgeries on the skull and maxillofacial regions. This tendency to control hypotension is because of the rich blood perfusion of these regions. Studies about hypotensive anesthesia for maxillofacial blood loss reported acceptable outcomes (21, 22), while information on the efficacy of this technique for craniosynostosis surgery is controversial and vague (23, 24).
2. Objectives
The present study was conducted to compare the effect of TXA, controlled hypotension, and TXA plus controlled hypotension anesthesia (CHA) on perioperative blood loss in craniosynostosis surgery.
3. Methods
3.1. Study Design and Participants
The present study was a double-blind, randomized clinical trial. The study population included all infants that were candidates for craniosynostosis correction surgery and were referred to Imam Hossein Hospital affiliated to Isfahan University of Medical Sciences, from April 2017 to June 2018. In this study, 75 patients were considered as the sample according to the sample size formula in between-groups comparison, at the confidence level of 95%, the test power of 80%, and considering the results of previous studies (25) reporting the mean and standard deviation of the total blood loss in control and TXA plus CHA groups equal to 1850.83 ± 800.73 ml and 1377.74 ± 423.46 ml, respectively.
Inclusion criteria consisted of patients that were candidates for craniosynostosis correction surgery, were within the age range of three to twelve months, and had the American Society of Anesthesiologists (ASA) score I or II. Moreover, patients with medical cardiac, hepatic, renal, and pulmonary diseases, a previous history of craniosynostosis surgery, and any other congenital abnormality did not meet the criteria. Exclusion criteria comprised the occurrence of any complications caused by anesthetics (e.g., hemodynamic instability) or surgical procedures (e.g., dura or cerebral venous sinuses rupture).
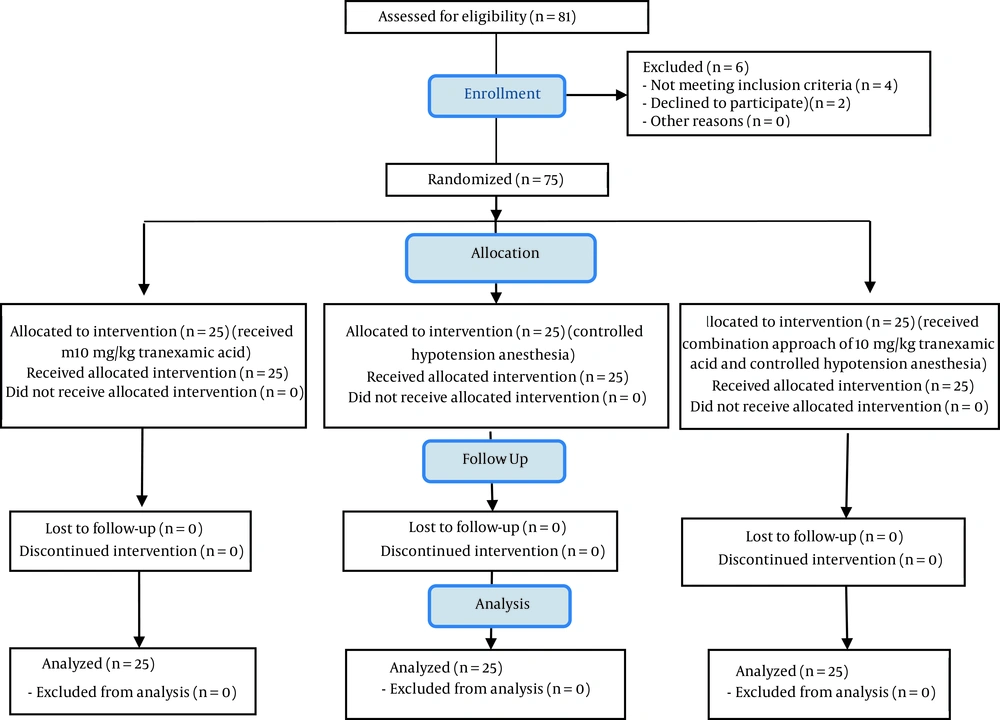
It should be noted that in this study, no patient suffered from the mentioned complications, as a result of which no decrease was reported in the sample size (Figure 1). After obtaining the code of ethics from the Ethics Committee of Isfahan University of Medical Sciences (approval code: IR.MUI.MED.REC.1396.3.597), the code of IRCT20190328043128N1 (link: www.irct.ir/ trial/43145) from Iranian Registry of Clinical Trials, and written consent from the infants’ parents, 75 eligible infants were randomly selected to enter the study. At the beginning of the study, patients’ basic information, including their age, gender, and weight was recorded. Then, the patients were divided into three groups using a random allocation software.
3.2. Intervention
An identical anesthesia protocol was performed for all infants in such a way that before the injection of anesthetic agents, two venous lines were taken from the infants. Blood transfusion was initiated by intravenous injecting 5 mg/kg sodium thiopental, 0.5 mg/kg of Atracurium, and 1 μ/kg fentanyl as anesthetic agents. The anesthesia was maintained by administering 1.2% MAC of isoflurane with oxygen and N2O in in equal proportions.
Ten minutes before the start of surgery, 10 mg/kg of TXA (Caspian Company, Iran) was administered intravenously to patients in the first group (TXA group). In the second group, patients were subjected to CHA using intravenous remifentanil 0.1 μ/kg (CHA group), which was repeated as needed during the surgical procedure so that the mean arterial pressure (MAP) was regulated at 30% below the MAP before anesthesia induction and within the range of 50 - 60 mmHg. In the third group, in addition to receiving 10 mg/kg of TXA intravenously (Caspian Company; Iran), the patients underwent CHA similar to that of the second group (CHA-TXA group). All studied medicines were administered in 20 cc dextrose water 5% serum for 15 minutes through intravenous infusion.
It is worth mentioning that to comply with blinding conditions, the anesthesiologist responsible for performing the interventions was not present in the operating room during the surgery, and another anesthesiologist, who had no knowledge of the type of intervention in each group, evaluated the patients’ condition and provided the required information.
3.3. Outcome Measures
Total perioperative blood loss is determined by measuring the blood collected in the suction bottle at the end of surgery and subtracting the volume of saline used for irrigation during the surgery, transfused blood volume (Packed red blood cells (PRBCs), and fresh frozen plasma (FFP)) during the surgery and in the intensive care unit, extubation time, and recovery time were recorded in the study checklist. It is necessary to mention that the maximum allowable blood loss was utilized to measure the maximum blood loss that can be considered acceptable for a patient during a surgical procedure (23). Injections of PRBCs compensated for this amount. Fresh frozen plasma was assessed for patients whose transfused blood volume exceeded 50% of their total blood volume.
The duration of surgery, the length of recovery stay, and the extubation time were recorded. Consider that extubation time expressed in terms of minutes was defined as the duration from anesthetic injection cessation to when the patient was wholly extubated. A Modified Aldrete Score was used to determine the recovery time (22). In addition, patients’ hemoglobin and hematocrit were recorded before and 6 hours after the surgery. Mean arterial pressure and heart rate (HR) were recorded before the surgery and every 15 minutes to 3 hours after the intervention (during and after the surgery).
3.4. Statistical Analysis
The obtained data were entered into SPSS-24 software (IBM® SPSS® Statistics; Chicago; The United States). Descriptive variables were presented as means and percentages. For analytics, one-way ANOVA (to compare the means of quantitative variables between groups), Tukey’s post hoc test (to perform pairwise comparisons between the means of quantitative variables), chi-squared test (to compare qualitative variables between groups), and repeated measures ANOVA (to evaluate the changes within one variable over time) were used. In all analyses, a significance level of less than 0.05 was considered.
4. Results
In the present study, the TXA group consisted of 14 (56%) girls and 11 (44%) boys with a mean age of 6.52 ± 3.00 months. The CHA group included 9 (36%) girls and 16 (64%) boys with a mean age of 8.00 ± 3.48 years. Moreover, the CHA-TXA group comprised 10 (40%) girls and 15 (60%) boys with a mean age of 8.40 ± 3.47 months (P-value > 0.05) (Table 1).
Abbreviations: TXA, tranexamic acid; CHA, controlled hypotensive anesthesia.
a Values are expressed as mean ± SD or No. (%).
b The significance level of the ANOVA test comparing the mean of the variables among the three groups.
c The significance level of the chi-squared test comparing the frequent distribution of the variable among the three groups.
Examination of hemodynamic parameters revealed that the three groups did not have significant differences in terms of MAP and HR at the beginning of the study (P-value > 0.05). However, these two parameters were significantly higher in the CHA-TXA group compared to the other two groups in follow-up times (P-value < 0.05). In addition, the changes in these two parameters over time (3 hours after surgery) were significant in all three groups; however, the lowest decrease was observed in the CHA-TXA group (P-value < 0.05) (Table 2).
| Parameters b | TXA Group (n = 25) | CHA Group (n = 25) | CHA-TXA Group (n = 25) | P c | P d | P e |
|---|---|---|---|---|---|---|
| MAP (mmHg) | ||||||
| T0 | 66.80 ± 10.03 | 66.00 ± 5.47 | 65.46 ± 8.16 | 0.728 | 0.606 | 0.784 |
| T15 | 62.40 ± 9.85 | 60.32 ± 5.75 | 62.17 ± 4.75 | 0.366 | 0.916 | 0.221 |
| T30 | 58.48 ± 6.09 | 57.32 ± 5.04 | 61.50 ± 5.50 | 0.467 | 0.072 | 0.007 |
| T45 | 57.24 ± 5.57 | 55.76 ± 4.67 | 60.88 ± 6.73 | 0.313 | 0.042 | 0.003 |
| T60 | 55.40 ± 6.06 | 54.72 ± 3.75 | 60.42 ± 6.26 | 0.635 | 0.006 | < 0.001 |
| T75 | 53.76 ± 5.71 | 53.04 ± 3.36 | 60.54 ± 8.97 | 0.590 | 0.002 | < 0.001 |
| T90 | 52.36 ± 5.70 | 52.80 ± 3.62 | 60.96 ± 10.06 | 0.746 | < 0.001 | < 0.001 |
| T105 | 53.65 ± 4.62 | 52.38 ± 4.08 | 61.29 ± 7.61 | 0.308 | < 0.001 | < 0.001 |
| T120 | 52.14 ± 4.79 | 51.05 ± 2.70 | 60.42 ± 5.16 | 0.326 | < 0.001 | < 0.001 |
| T135 | 53.12 ± 6.41 | 51.07 ± 2.70 | 62.13 ± 5.53 | 0.147 | < 0.001 | < 0.001 |
| T150 | 51.91 ± 3.53 | 50.13 ± 1.72 | 61.25 ± 4.97 | 0.028 | < 0.001 | < 0.001 |
| T165 | 51.25 ± 3.77 | 49.80 ± 1.78 | 62.00 ± 4.89 | 0.088 | < 0.001 | < 0.001 |
| T180 | 49.50 ± 3.70 | 52.00 ± 5.65 | 60.67 ± 5.50 | 0.070 | < 0.001 | 0.011 |
| P f | < 0.001 | < 0.001 | 0.014 | |||
| HR (bpm) | ||||||
| T0 | 144.88 ± 16.42 | 143.08 ± 9.40 | 137.67 ± 21.22 | 0.636 | 0.185 | 0.249 |
| T15 | 136.04 ± 19.93 | 123.48 ± 9.72 | 134.92 ± 16.37 | 0.006 | 0.829 | 0.005 |
| T30 | 130.96 ± 17.85 | 121.48 ± 9.81 | 134.92 ± 18.34 | 0.024 | 0.442 | 0.003 |
| T45 | 129.64 ± 20.71 | 119.56 ± 9.22 | 135.42 ± 17.90 | 0.031 | 0.296 | < 0.001 |
| T60 | 128.92 ± 20.97 | 119.04 ± 8.72 | 135.21 ± 16.91 | 0.034 | 0.249 | < 0.001 |
| T75 | 123.88 ± 13.16 | 117.16 ± 9.97 | 135.33 ± 16.68 | 0.047 | 0.009 | < 0.001 |
| T90 | 123.64 ± 14.82 | 116.64 ± 9.76 | 135.58 ± 15.84 | 0.054 | 0.008 | < 0.001 |
| T105 | 121.43 ± 14.44 | 114.79 ± 8.35 | 132.95 ± 13.65 | 0.052 | 0.006 | < 0.001 |
| T120 | 121.27 ± 14.99 | 114.14 ± 8.36 | 132.21 ± 12.51 | 0.043 | 0.007 | < 0.001 |
| T135 | 124.53 ± 14.65 | 114.71 ± 6.46 | 133.50 ± 12.87 | 0.003 | 0.025 | < 0.001 |
| T150 | 124.27 ± 18.89 | 113.38 ± 6.25 | 132.17 ± 10.43 | 0.008 | 0.043 | < 0.001 |
| T165 | 133.50 ± 29.85 | 110.20 ± 4.43 | 129.50 ± 6.62 | < 0.001 | 0.516 | < 0.001 |
| T180 | 133.50 ± 51.61 | 106.00 ± 60.00 | 127.67 ± 2.51 | 0.088 | 0.575 | 0.077 |
| P f | < 0.001 | < 0.001 | 0.038 |
Abbreviations: TXA, tranexamic acid; CHA, controlled hypotensive anesthesia; MAP, mean arterial pressure; HR, heart rate.
a Values are expressed as mean ± SD.
b T0: Baseline, T15: 15 minutes after intervention, T30: 30 minutes after intervention, T45: 45 minutes after intervention, T60: 60 minutes after intervention, T75: 75 minutes after intervention, T90: 90 minutes after intervention, T105: 105 minutes after intervention, T: 120 minutes after intervention, T135: 135 minutes after intervention, T150: 150 minutes after intervention, T165: 165 minutes after intervention, T180: 180 minutes after intervention.
c The significance level obtained from Tukey’s post hoc test to compare the variable mean of tranexamic acid group with that of controlled hypotension anesthesia group.
d The significance level obtained from Tukey’s post hoc test to compare the variable mean of tranexamic acid group with that of controlled hypotension anesthesia-tranexamic acid group.
e The significance level obtained from Tukey’s post hoc test to compare the variable mean controlled hypotension anesthesia group with that of controlled hypotension anesthesia-tranexamic acid group.
f The significance level obtained from the repeated measures ANOVA test to compare the variable mean in each of the groups over time (time effect).
Analysis of data related to surgery indicated that the total perioperative blood loss in the CHA-TXA group with the mean of 181.20 ± 82.71 cc was significantly less than the total perioperative blood loss in the CHA and TXA groups with the means of 262.00 ± 104.04 cc and 212.80 ± 80.75 cc, respectively (P-value < 0.05). Furthermore, the transfusion volume in the CHA-TXA group with the mean of 112.40 ± 53.50 cc was significantly lower than the transfusion volume in the CHA and TXA groups with the means of 174.00 ± 73.93 cc and 160.63 ± 59.35 cc, respectively (P-value < 0.05). In contrast, the total blood loss and transfusion volume were not significantly different between CHA and TXA groups (P-value > 0.05). In addition, the pairwise comparison of the two groups showed that the patients’ hematocrit and hemoglobin before and 6 hours after the surgery had no significant difference with each other (P-value > 0.05). However, over time, the hemoglobin and hematocrit levels in the CHA group and only the hematocrit level in the TXA group had a significant decrease within 6 hours after surgery (P-value < 0.05) (Table 3).
| Variables | TXA Group (n = 25) | CHA Group (n = 25) | CHA-TXA Group (n = 25) | P b | P c | P d |
|---|---|---|---|---|---|---|
| Hemoglobin | ||||||
| Before surgery | 11.62 ± 0.86 | 11.33 ± 1.10 | 13.15 ± 5.63 | 0.304 | 0.185 | 0.119 |
| 6 hours after surgery | 11.14 ± 1.41 | 10.78 ± 0.84 | 11.20 ± 2.35 | 0.278 | 0.913 | 0.404 |
| P e | 0.051 | 0.004 | 0.085 | |||
| Hematocrit | ||||||
| Before surgery | 35.05 ± 2.50 | 34.00 ± 3.30 | 39.53 ± 16.90 | 0.211 | 0.196 | 0.115 |
| 6 hours after surgery | 33.24 ± 3.92 | 32.34 ± 2.52 | 33.40 ± 6.87 | 0.339 | 0.920 | 0.472 |
| P e | 0.006 | 0.004 | 0.069 | |||
| Total blood loss during surgery (cc) | 212.80 ± 80.75 | 262.00 ± 104.04 | 181.20 ± 82.71 | 0.067 | 0.038 | 0.004 |
| Transfusion volume (cc) | 160.63 ± 59.35 | 174.00 ± 73.93 | 112.40 ± 53.50 | 0.484 | 0.004 | 0.001 |
| Duration of surgery (min) | 136.04 ± 24.49 | 134.40 ± 25.05 | 139.60 ± 31.38 | 0.816 | 0.657 | 0.520 |
| Length of recovery stay (min) | 72.80 ± 15.88 | 78.00 ± 12.50 | 80.20 ± 27.51 | 0.204 | 0.249 | 0.717 |
| Extubation time (min) | 18.00 ± 9.89 | 23.20 ± 8.64 | 24.79 ± 14.48 | 0.053 | 0.051 | 0.639 |
Abbreviations: TXA, tranexamic acid; CHA, controlled hypotensive anesthesia.
a Values are expressed as mean ± SD.
b The significance level obtained from Tukey’s post hoc test to compare the variable mean of tranexamic acid group with that of controlled hypotension anesthesia group.
c The significance level obtained from Tukey’s post hoc test to compare the variable mean of tranexamic acid group with that of controlled hypotension anesthesia-tranexamic acid group.
d The significance level obtained from Tukey’s post hoc test to compare the variable mean controlled hypotension anesthesia group with that of controlled hypotension anesthesia-tranexamic acid group.
e The significance level obtained from the repeated measures ANOVA test to compare the variable mean in each of the groups over time (time effect).
5. Discussion
The study findings showed that the three groups were similar in terms of age, sex distribution, and body weight. In addition, the duration of surgery, the length of recovery stay, and the extubation time were not statistically different among the three groups. Therefore, it seems that confounding variables did not affect the results of this study. Total blood loss and transfusion volume were significantly lower in the CHA-TXA group compared with the other two groups. Moreover, the means of total blood loss and transfusion volume were significantly lower in the TXA group compared with the CHA alone group. In addition, Hb and HCT changes in the CHA-TXA group were lower than Hb and HCT changes in the other two groups; however, in general, the three groups did not have significant differences with each other in terms of the mean of these two factors.
In line with the findings of this study, the results of Zhang et al.’s study also indicated the effectiveness of the combination of CHA and TXA in reducing blood loss and transfusion after the surgery such that the total blood loss and blood transfusion rates in this approach were approximately 30% and 15%, respectively (25). In addition, they stated that Hb and HCT levels were better maintained by administering TXA plus CHA (25). In fact, a faster recovery indicated by the reduced length of hospital stay in the two CHA groups was experienced after the surgery by improving the HCT and Hb levels. Contrary to the antiplasmin ability of TXA, hemorrhage was reduced by HA as a result of changing the regional tissue perfusion during surgery (26). The literature acknowledges that intraoperative blood loss could be significantly reduced by HA up to 30 - 50% (27, 28). It can be stated that the effects of CHA can be directly combined with TXA. In addition, as hypotensive status can be maintained for several hours after recovery from anesthesia, rebound bleeding can be avoided following the fading away of spillover hypotensive effects. Moreover, the muscle and bone surface bleeding constantly reduces and, in turn, decreases dominant blood loss following the surgery (29).
Moreover, the antifibrinolytic effect of TXA and the inhibition of plasmin generation can also be effective in controlling blood loss. In other words, TXA seems to effectively inhibit fibrinolysis caused by surgical trauma, while CHA is mainly effective during surgery. The role of CHA in blood loss has been poorly in previous studies (30, 31).
Many studies have reported the successful use of TXA to reduce perioperative blood loss and PRBC transfusion in pediatric surgeries. Although this agent has been effectively used in orthopedic surgeries (32), there was a major concern about whether TXA can successfully control blood-related complications of cranial surgeries, especially craniosynostosis (7, 33). In this regard, numerous studies were performed and are in progress, indicating successful outcomes of TXA for controlling blood loss during craniosynostosis surgery (1, 7, 34-36), which is consistent with our findings. Diarrhea, nausea, orthostatic reactions, and hypersensitivity are adverse effects of TXA and rarely have been focused on (37, 38). Thrombotic incidents that can be attributed to the nature of TXA are the most concerning complication of this agent and have not been addressed in the literature (32, 39).
However, the findings of our study indicated that patients’ MAP and HR were more stable in the CHA-TXA group compared with the other two groups over 180 minutes. Moreover, the TXA group was more successful than the CHA group alone in this respect. Barak et al. addressed the efficacy of hypotensive anesthesia in major maxillofacial surgeries and concluded that concise patient selection for hypotensive anesthesia and adequate blood loss replacement are necessary to successfully and safely perform this approach during major maxillofacial surgeries (22).
In a more recent study, Shaban et al. presented the efficacy of hypotensive anesthesia during maxillofacial surgeries though they also presented probable complications (21). Fearon et al. performed a study assessing hypotensive anesthesia during CVR for craniosynostosis surgery, compared it with normotensive anesthesia, and reported no difference (24). They eventually claimed that the lack of a significant difference between the two groups shows that hypotensive anesthesia is not worth controlling blood loss and transfusion during craniosynostosis surgery among this vulnerable population (24). These outcomes were presented by Chang et al. in a study on patients that underwent craniosynostosis surgery from 2013 to 2016 (40). The findings of another study ascribed the mentioned finding to the quite mild hemodynamic micro-environment provided by the hypotension status. This micro-environment may improve clot formation and stabilization, which in turn facilitate the effects of TXA (29). However, further studies are required to shed more light on the issue.
In particular, there has been no study evaluating the effect of the combination of TXA and CHA on the blood loss control and perioperative blood transfusion in craniosynostosis surgery. Therefore, in comparison with other studies, caution should be given due to the difference in the type of surgery and the age range of patients. The limitations of this study may include the small sample size, the non-consideration of blood on the surgical instruments or blood gases in the calculation of total blood loss, and the lack of any separate reports regarding the frequency of using blood products, including PRBC, FFP, and platelets. However, attention to two conventional methods of blood loss control in this rare and high-risk surgery can be considered one of the strengths of this study. Considering the special conditions of this surgery and the patients’ high-risk age range (children under one year), it seems necessary to conduct more studies in this regard to achieve more accurate, reliable, and generalizable results.
5.1. Conclusions
Based on the results of the present study, although the administration of TXA alone could effectively prevent blood loss and was associated with fewer transfusion requirements, the combination of this approach with hypotensive anesthesia resulted in not only more favorable results in reducing perioperative blood loss and transfusion volume but also better hemodynamic stability.