1. Background
Pain is one of the medical problems frequently misdiagnosed, undertreated, and poorly understood, particularly in children. Poor pain management during childhood may have long-term deleterious impacts, including damaging neuro-endocrine reactions, disturbed eating and sleeping patterns, and increased pain sensitivity during subsequent painful events (1, 2).
The caudal epidural block is the most common regional approach utilized in hypospadias repair procedures since it leads to a less stressful recovery with earlier ambulation, reduced risk of chest infections, less postoperative painkiller use, and earlier release (3, 4). Despite this, many parents still disregard caudal anesthesia (CA), which may result in a rare neurological consequence (5).
Dexmedetomidine is an alpha-2 agonist with sedative, analgesic, and opioid-sparing properties. It extends the endurance of the analgesic effect by having a local vasoconstrictive impact and boosting potassium conductance in A-delta and C-fibers (6). It has a central analgesic effect, which is reaching alpha 2 receptors in the superficial laminae of the spinal cord and brainstem or indirectly stimulating spinal cholinergic neurons by systemic absorption or diffusion into the cerebrospinal fluid. The main mechanism underlying the sedative effects of dexmedetomidine is activating the alpha 2 adreno-receptor in the locus coeruleus (7, 8).
When used as a part of Enhanced Recovery After Surgery regimens, perioperative dexmedetomidine administration has considerably improved postoperative results (9).
2. Objectives
This study compared intraoperative/intravenous dexmedetomidine infusion to the caudal block in pediatric patients undergoing hypospadias correction surgery to determine their effectiveness and safety. Our primary outcome was assessing the patients’ quality of recovery as measured by the modified objective pain score (MOPS), and secondary outcomes included postoperative analgesic requirements, postoperative sedation, time to extubation, and the incidence of any perioperative complication.
3. Methods
This prospective open-label trial, randomized study was carried out in the pediatric surgery department from March 2020 to October 2020 on 90 male children, American Society of Anesthesiologists (ASA) physical status I and II, who were aged 2 - 12 years and scheduled for hypospadias repair surgery. This study was approved by the Research Ethics Committee of the Tanta University (Code: 33681/2/20).
After obtaining approval from the Ethics Committee, the study was registered on ClinicalTrials.gov (ID: NCT04331418), and written informed consent was obtained from the children’s parents. Each parent received information about the research objectives and had a secret code number, and photos were only from the body parts associated with the research to protect the participants’ privacy and observe information confidentiality.
The following patients were excluded from the trial: Patients having back infections, allergies to the study medicines, bleeding, and coagulation disorders, and those with a history of developmental delays, sepsis, or pre-existing neurological or spinal abnormalities.
Preanesthetic checkups and routine investigations were performed for each patient. According to the ASA’s recommendation on fasting, the patients maintained nil by mouth. Moreover, the baseline heart rate (HR), mean arterial pressure (MAP), and peripheral oxygen saturation (SpO2) were measured when the patients entered the operating room.
Further, O2/air/sevoflurane, fentanyl 0.5 - 1 μg/kilogram (kg), and cisatracurium 0.15 milligrams (mg)/kg IV were used to induce general anesthesia. The trachea was intubated using the proper endotracheal tube, and anesthesia was maintained using O2/air, sevoflurane 2%, increments of fentanyl 0.5 μg/kg, and cis-atracurium 0.03 mg/kg intravenously, as determined by hemodynamics and capnography.
The patients were randomly divided into three equal groups. Randomization was performed using the sealed opaque envelope technique; each patient’s parent randomly selected a sealed envelope containing a group number to which the patient was assigned. Group I (the control group (Group C)) received general anesthesia. Group II (caudal group) underwent a caudal block using a 25-gauge needle inserted through the sacral hiatus between the bilateral sacral cornua to enter the sacral epidural space. This procedure was performed with the patient in the left lateral position under strict aseptic and antiseptic conditions. The pop felt during sacrococcygeal ligament penetration and the whoosh test (10), which used 0.5 ml of air to validate the needle’s position. When no blood or cerebrospinal fluid was aspirated, we administered 2.5 mg/kg of bupivacaine at a dose of 0.5 ml/kg (concentration 0.5%). The injection site was subsequently dressed, the patients were turned supine, and the surgical process began 15 min from the caudal block.
Dexmedetomidine was given intravenously (IV) to children in Group III (the dexmedetomidine group (Group D)) at a dose of 1 μg/kg over 10 minutes, followed by 0.5 μg/h, with a recommended maximum dose of 2 mg/kg (Precedex, manufactured by Abbott Laboratories in North Chicago, Illinois, is available in a 2 mL preservative-free vial at a concentration of 100 μg/mL (normal saline solution, 2 to 4 μg/mL)) (11).
For all groups, ondansetron 0.15 mg/kg IV as postoperative nausea and vomiting (PONV) prophylaxis and paracetamol 15 mg/kg iv infusion were given 10 minutes before the procedure. Postoperative rescue analgesia was given in the form of a diclofenac 1 mg/kg suppository.
At the end of the operation, dexmedetomidone was stopped in the dexmetomidine group, and all children were extubated when respiration was deemed sufficient, and they could obey commands and transferred to the recovery room. In the postanesthesia care unit (PACU), they were monitored for any evidence of complications or adverse events and discharged when the alert score was ≥ 9. The parents were allowed to be with their children.
In the two groups, the particiapants’ age and duration of the surgery were recorded. Using Minogue et al.’s three-category scale, we evaluated the severity of coughs; one cough was classified as mild, continuous coughs lasting for five seconds or less were moderate, and continuous coughs lasting above five seconds were classified as severe (12).
A nurse who was not involved in the research design evaluated the modified objective pain score (MOPS) (13) 30 minutes, and one, two, four, eight, and 24 hours after surgery. There are five parameters in the MOPS: Crying (C) (0 = none, 1 = consolable, 2 = inconsolable), movements (M) (0 = none, 1 = restless, 2 = thrashing), agitation (A) (0 = asleep or calm, 1 = mild, 2 = hysterical), posture (P) (0 = normal, 1 = flexed, 2 = holds injury site), and verbal signs (0 = asleep or not complaining, 1 = complaint but cannot localize, 2 = complaint but can localize). If MOPS > four, the patient was given an additional IV injection of pethidine (0.5 mg/kg IV) as a form of rescue analgesia.
The Ramsay sedation scale (14) was used to gauge the degree of sedation 15, 30, and 60 minutes after extubation. Thereafter, it was used on an hourly basis until all patients had a Ramsay sedation score of one.
Time to extubation, total amount of pethidine during the first 24 hours after the operation, and complications such as bradycardia (if HR is < 20% of baseline), hypotension (decrease in basal MAP by 20%), respiratory depression (the SpO2 is < 95%), need for O2 supplementation, and postoperative nausea and vomiting, were also recorded.
3.1. Statistical Analysis
Our primary outcome was MOPS. According to the results of a previous study, the sample size was estimated to be 42 patients per group to detect a significant difference in MOPSs of 0.3 with a standard deviation of 0.67 at α error of 0.05 with the research power of 80% (15). We assigned 45 patients to group to overcome possible dropouts. Data were imported to the computer and analyzed with IBM SPSS software package version 22 (Armonk, NY: IBM Corp). Categorical data were represented as frequencies and percentages. The chi-square test was run to investigate the relationship between the categorical variables. Alternatively, Fisher’s Exact or Monte Carlo correction test was used when the expected cell counts were < five. For continuous data, the Shapiro-Wilk test was used to test normality. Distributed data were expressed as a range (minimum and maximum), mean, standard deviation, and median. Student t-tests were used for normally distributed quantitative variables. On the other hand, the Mann-Whitney U test was used for non-normally distributed quantitative variables. The significance level in this study was set to be P = 0.05.
4. Results
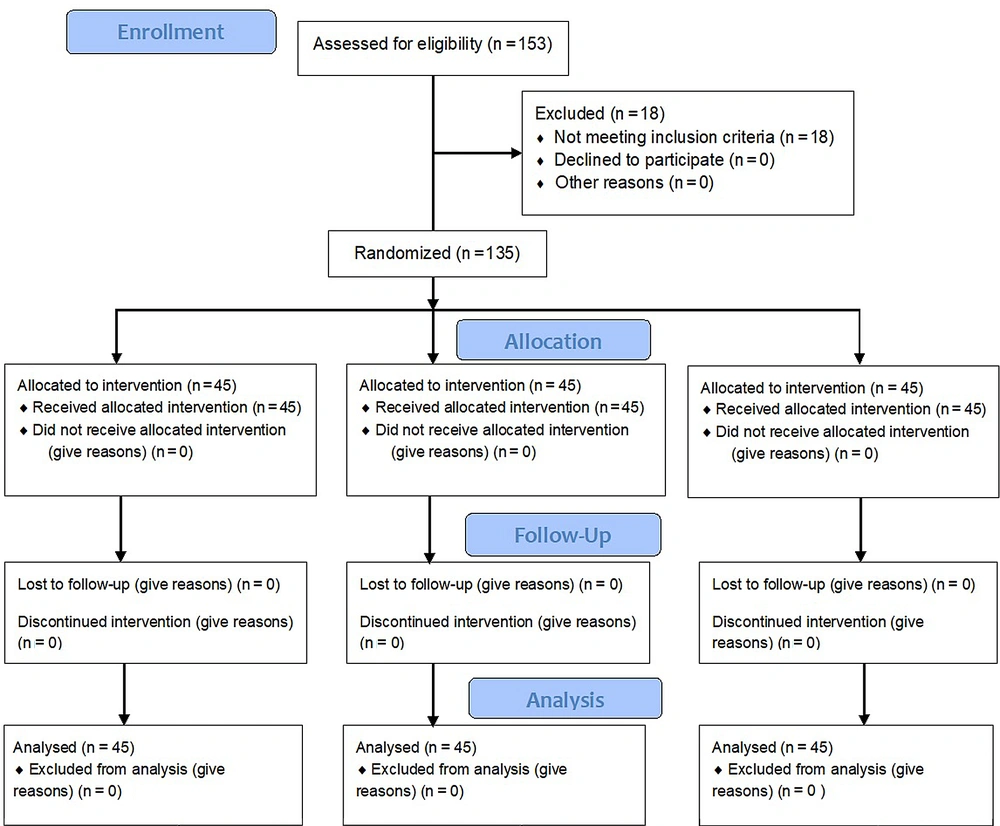
One hundred and fifty-three patients were assessed for eligibility, of whom 18 persons were excluded due to parental refusal, and 135 patients were finally analyzed and equally assigned into three groups (n = 45 per group) (Figure 1).
Both groups were comparable in terms of demographic data, including age and surgical duration (P = 0.719 and 0.184, respectively). Regarding postoperative pethidine dose, the patients who received GA required a higher dose than the caudal and Group D (2.3 ± 0.7 vs. 0.5 ± 0.3 and 0.7 ± 0.3 mg/kg, respectively) at P < 0.001 for both groups, with no significant difference between caudal and dexmedetomidine (P = 0.087). Our results showed that the patients receiving dexmedetomidine were extubated significantly later than those in the control and caudal groups (14.8 ± 1.4 minutes vs. 11.5 ± 1.2 and 6.9 ± 0.8, respectively; P < 0.001) (Table 1).
| Variables | Control (n = 45) | Caudal (n = 45) | Dexmedetomidine (n = 45) | P-Value |
|---|---|---|---|---|
| Age (y) | 0.719 | |||
| Mean ± SD | 6.1 ± 1.6 | 6.3 ± 1.8 | 6.4 ± 1.7 | |
| Median (min - max) | 5 (4 - 10) | 5 (4 - 10) | 6 (4 - 10) | |
| Surgical duration (min) | 0.184 | |||
| Mean ± SD | 122.6 ± 3.8 | 124.1 ± 5.3 | 124.2 ± 4.9 | |
| Median (min - max) | 120 (115 - 130) | 125 (115 - 135) | 125 (115 - 135) | |
| Time to extubation (min) | < 0.001 a | |||
| Mean ± SD | 11.5 ± 1.2 | 6.9 ± 0.8 | 14.8 ± 1.4 | |
| Median (min - max) | 12 (9 - 14) | 7 (6 - 9) | 15 (11 - 18) | |
| Pethidine dose (mg) | < 0.001 a | |||
| Mean ± SD | 2.3± 0.7 | 0.5 ± 0.3 | 0.7 ± 0.3 | |
| Median (min - max) | 2.3 (1.2 - 3.5) | 0.5 (0 - 0.8) | 0.8 (0 - 1.7) |
a Statistically significant at P ≤ 0.05.
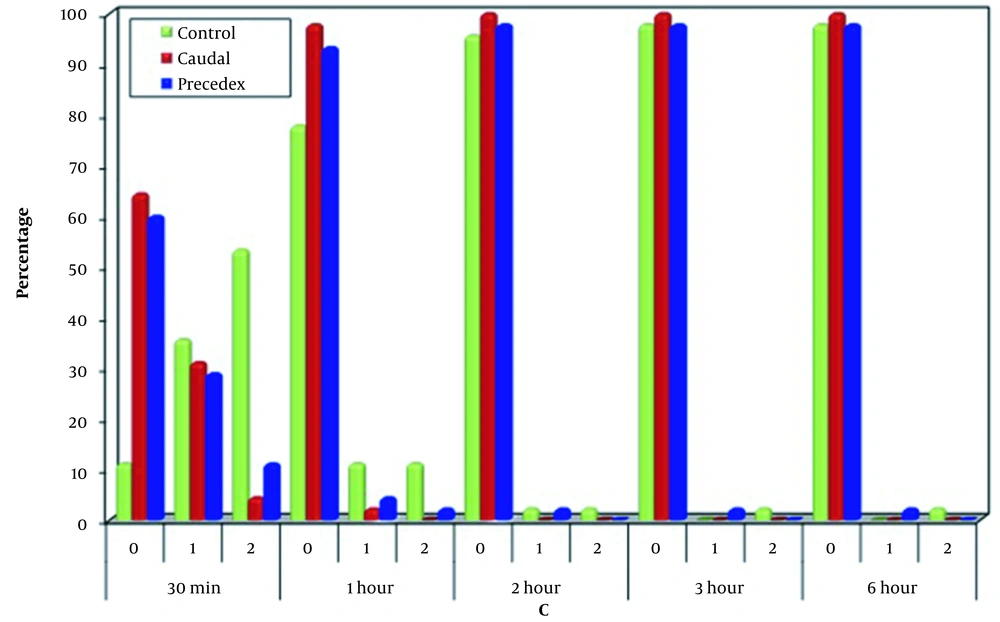
Regarding MOPSs for C 30 minutes after operation, most cases in Group C had non-consolable crying (53.3%), while most cases in the caudal and (64.4%) and dexmedetomidine (60%) groups did not cry; however, there was a significant difference between the control and other two groups (P < 0.001). On the other hand, no significant difference was observed between the caudal and dexmedetomidine groups (P = 0.642). One, two, three, and six hours after operation, the three groups were comparable in terms of the crying component of MOPS (Figure 2).
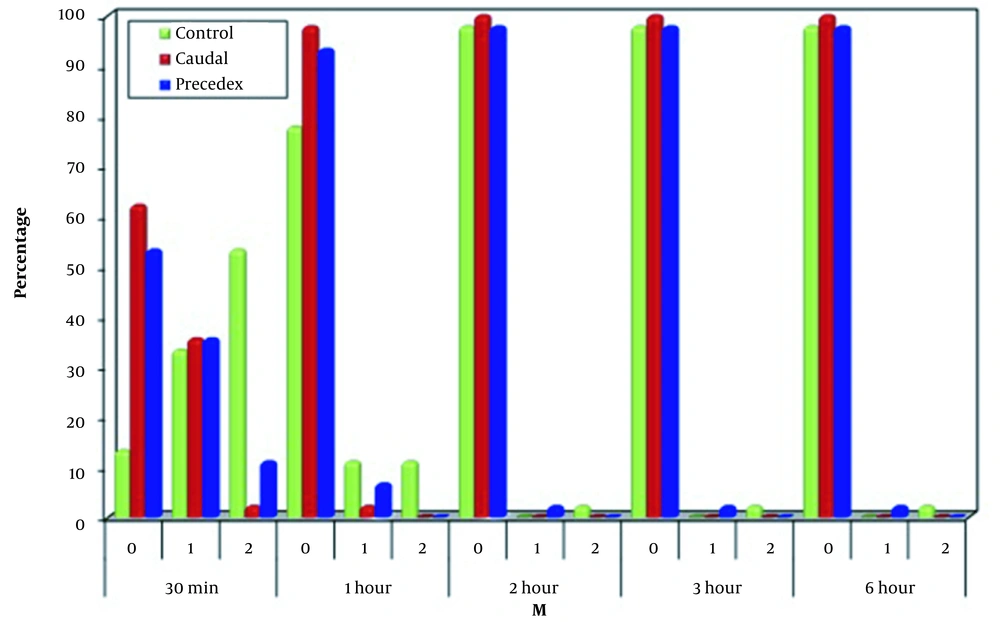
Regarding M, a significant difference was noticed among the three groups 30 minutes and one hour after operation, as most cases in Group C had thrashing movement (53.3%), which was significantly higher than the other two groups (P < 0.001 for both groups). However, one hour after operation, 77.8% of Group C had no movement, which was significantly lower than the caudal and dexmedetomidine groups (P = 0.01 and 0.044, respectively). Thirty minutes and one hour after operation, there was no significant difference between the caudal and dexmedetomidine groups regarding movements. The three groups were significantly similar at two, three, and six hours regarding the movements (Figure 3).
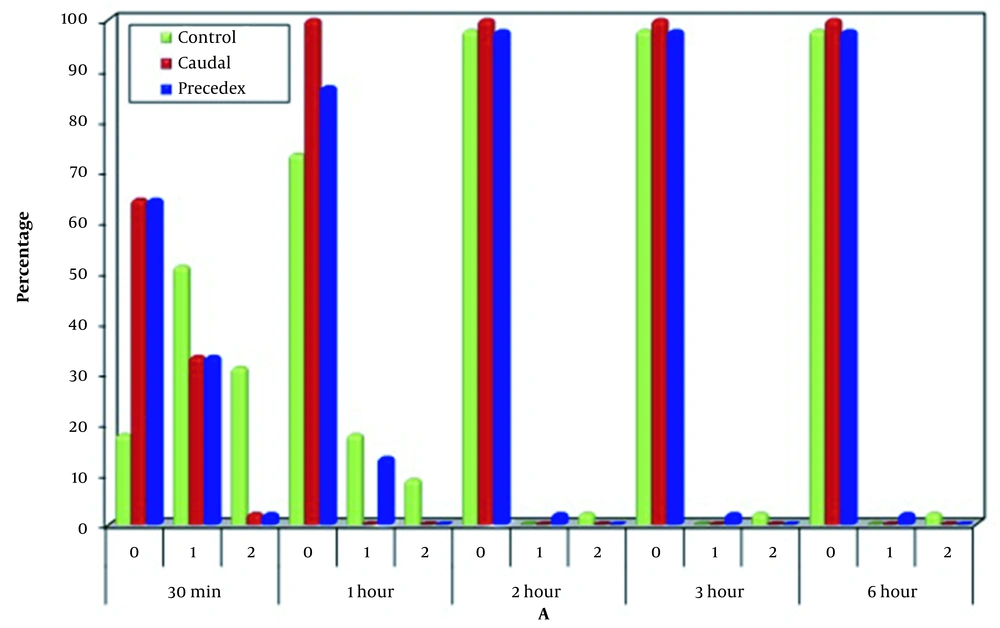
Regarding A, a significant difference was noticed among the three groups 30 minutes and one hour after operation, as the majority of cases in Group C had mild agitation (51.1%), which was significantly higher than the other two groups (P < 0.001 for both groups). However, one hour after operation, (73.3%) of Group C were calm, which was significantly lower than the caudal group (P < 0.001). However, a significant difference was observed when compared to Group Ds (P = 0.096). The three groups were significantly similar at two, three, and six hours after operation regarding the agitation (Figure 4).
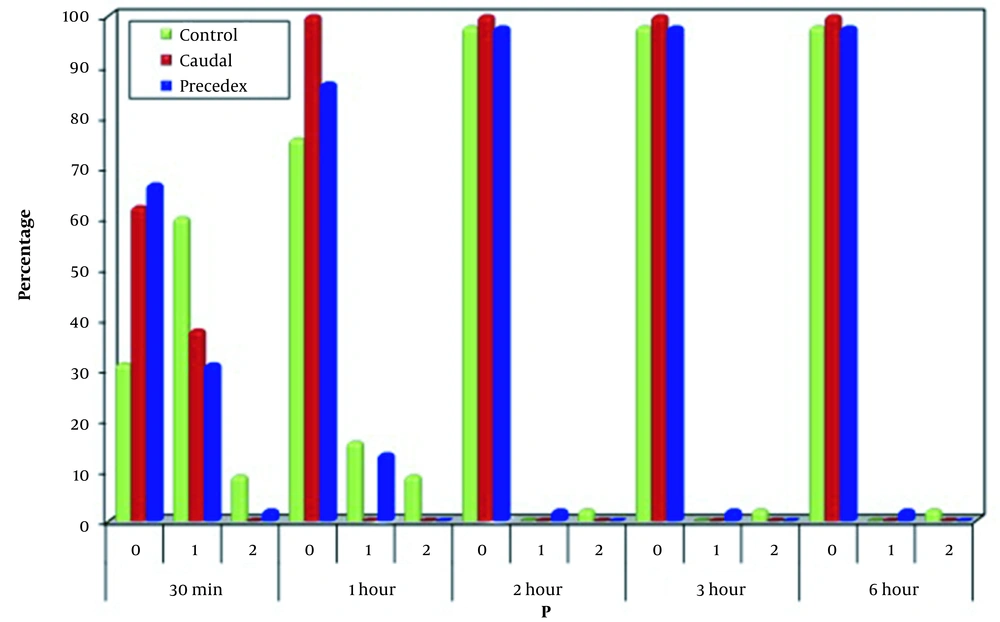
Regarding P, a significant difference was noticed among the three groups 30 minutes and one hour after operation, as many cases in Group C (60.0%) were flexed while 62.2 and 66.7% of the caudal and dexmedetomidine groups was normal 30 minutes after operation, respectively. One hour after the operation, 75.6% of the patients in Group C, 100% of the patients in the caudal group, and 86.7% of the patients in Group D were normal. The three groups were significantly similar regarding two, three, and six hours after the operation regarding posture. On the other hand, the groups were significantly similar at 30 minutes, one, two, three, and six hours after the operation regarding the verbal signs (Figures 5 and 6).
Assessing postoperative sedation by the Ramsay scale showed non-significant differences among the groups (Table 2).
| Assessment Time | Control (n = 45) | Caudal (n = 45) | Dexmedetomidine (n = 45) | P-Value | |
|---|---|---|---|---|---|
| Ramsay | 15 min | ||||
| Range | 1 (1 - 2) | 1 (1 - 2) | 1 (1 - 2) | 0.663 | |
| 1 | 33 (73.3) | 31 (68.9) | 29 (64.4) | 0.661 | |
| 2 | 12 (26.7) | 14 (31.1) | 16 (35.6) | ||
| 30 min | |||||
| Range | 2 (1 - 2) | 2 (1 - 2) | 2 (1 - 2) | 0.188 | |
| 1 | 11 (24.4) | 6 (13.3) | 5 (11.1) | 0.186 | |
| 2 | 34 (75.6) | 39 (86.7) | 40 (88.9) | ||
| 60 min | |||||
| Range | 2 (1 - 2) | 2 (2 - 2) | 2 (2 - 2) | 0.133 | |
| 1 | 2 (4.4) | 0 (0) | 0 (0) | 0.333 | |
| 2 | 43 (95.6) | 45 (100) | 45 (100) | ||
| 2 h | |||||
| Range | 2 (1 - 2) | 2 (2 - 2) | 2 (2 - 2) | 0.368 | |
| 1 | 1 (2.2) | 0 (0) | 0 (0) | 1.000 | |
| 2 | 44 (97.8) | 45 (100) | 45 (100) |
a Values are expressed as median (min - max) or No (%).
Regarding the incidence of complications, cough, bradycardia, hypotension, hypertension, respiratory depression, and PONV were comparable among the three groups. On the other hand, most of the cases in Group C developed shivering postoperatively, with no significant difference when compared to the caudal and dexmedetomidine groups at P = 0.306. Regarding tachycardia, a significantly larger number of patients in Group C (66.7%) developed tachycardia when compared to the caudal and dexmedetomidine groups (20% and 22.2%, respectively, P < 0.001) (Table 3).
| Research Parameters | Control (n = 45) | Caudal (n = 45) | Dexmedetomidine (n = 45) | P-Value |
|---|---|---|---|---|
| Cough | 0.362 | |||
| No | 32 (71.1) | 38 (84.4) | 39 (86.7) | |
| Moderate | 11 (24.4) | 6 (13.3) | 5 (11.1) | |
| Severe | 2 (4.4) | 1 (2.2) | 1 (2.2) | |
| Shivering | 10 (22.2) | 6 (13.3) | 5 (11.1) | 0.306 |
| Bradycardia | 2 (4.4) | 8 (17.8) | 9 (20) | 0.072 |
| Tachycardia | 30 (66.7) | 9 (20) | 10 (22.2) | < 0.001 b |
| Hypotension | 1 (2.2) | 7 (15.6) | 4 (8.9) | 0.093 |
| Hypertension | 19 (42.2) | 10 (22.2) | 11 (24.4) | 0.075 |
| Respiratory depression | 4 (8.9) | 5 (11.1) | 5 (11.1) | 1.000 |
| POV | 0.692 | |||
| No | 43 (95.6) | 44 (97.8) | 42 (93.3) | |
| Yes | 2 (4.4) | 0 (0) | 2 (4.4) | |
| Severe | 0 (0) | 1 (2.2) | 1 (2.2) |
a Values are expressed as No (%).
b Statistically significant at P ≤ 0.05
5. Discussion
Recovery from procedures to treat hypospadias is quite painful. With few downsides, CA is the most widely used, reliable, and safe regional approach in pediatric analgesia for such scenarios. However, high-concentration local anesthetic injections can raise the risk of motor weakness, delayed micturition, or urinary retention (16, 17). This randomized prospective study focused on the recovery and analgesic characteristics of intravenous dexmedetomidine when compared to CA and general anesthesia in pediatric patients undergoing hypospadias surgery repair.
Until now, no study has discussed the effects of the caudal block versus IV dexmedetomidine on improving the quality of pain and emergency after hypospadias repair in detail.
In this study, postoperative pethidine dose was higher in patients receiving GA than in the caudal and Group Ds, with no significant difference between the caudal and dexmedetomidine groups. Moreover, patients receiving dexmedetomidine were extubated significantly later than those in the control and caudal groups.
Regarding MOPS, crying, movements, postures, and agitation, this study showed a significant difference between Group C and the other two groups 30 minutes and one hour after operation for movements, postures, and agitation.
Following Li et al., 90 patients undergoing elective open gastrectomy under TIVA in this study were divided into three groups: The control group received a placebo, the epidural group (Group E) received epidural anesthesia, and Group D received IV dexmedetomidine 0.6 μg/kg before the induction of general anesthesia, followed by dexmedetomidine 0.4 μg/kg/h until peritoneal closure (18). Group D had a lower agitation rate (6.7%) that Group C (26.6%) (P = 0.038). In contrast to our findings, Li et al. found that the time of tracheal extubation was the same across all groups (18).
Ninety children were randomly divided into three groups and given normal saline (Group S), dexmedetomidine 0.5 μg/kg (Group D 0.5), or dexmedetomidine 1 μg/kg (Group D1). Bhat et al. evaluated the effectiveness of two doses of dexmedetomidine on laryngeal mask airway removal (19). They reported that dexmedetomidine 1 μg/kg was more effective than 0.5 μg/kg in reducing emergence agitation (19).
Our findings are consistent with those reported by Guler et al., who carried out a comparable trial on children and similarly concluded that dexmedetomidine 0.5 mg/kg facilitates smooth extubation and lessens agitation following sevoflurane anesthesia in children with adenotonsillectomy (20). We discovered that the time to emergence was substantially longer in the dexmedetomidine 0.5 μg/kg group (9.30 ± 2.9 min) compared to the placebo group (7.20 ± 2.7 min) (20). Similar findings were presented in our study, which can be explained by the sedative effects of dexmedetomidine. Kim et al. also concluded that intraoperative dexmedetomidine administration at 0.4 μg/kg provided stable hemodynamic emergence (21).
Furthermore, Bindu et al. documented that dexmedetomidine 0.5 μg/kg considerably decreased the emergence time and recovery time, hypothesizing that a reduction in the intraoperative need for sevoflurane might have caused an earlier waking (22).
Yang et al. concluded that dexmedetomidine enhances recovery quality and reduces emergence agitation in their systematic review and meta-analysis on the effects of dexmedetomidine on decreasing emergence agitation in children following various types of procedures under general anesthesia (23).
Many studies support the analgesic benefits of dexmedetomidine. Children with an intraoperative dexmedetomidine infusion after hypospadias correction surgery had considerably reduced pain and behavior scores in the PACU, according to Patel et al., as both the number of patients in the PACU who needed morphine and asked for it significantly decreased (24).
Dexmedetomidine was found to be more effective than fentanyl in reducing postoperative pain scores and the number of rescue doses of morphine in a trial on children with obstructive sleep apnea after tonsillectomy and adenoidectomy. Even after surgery, dexmedetomidine’s analgesic and opioid-sparing effects were still clearly observed; the patients receiving dexmedetomidine required analgesics much later in the course of treatment (25).
When evaluating postoperative agitation in children with lumbosacral spinal dysraphism, who were randomly randomized to receive either dexmedetomidine or a placebo, Gupta et al. reached a different finding (26). There was no appreciable variation between the two groups’ emergence and extubation time. However, postoperatively, the kids in Group D had markedly lower pain levels and agitation scores, coupled with a more extended period of initial analgesic necessity, which was consistent with the present findings (26).
Inconsistent findings are re reported in the literature on delays in the emergence and prolongation of extubation time when dexmedetomidine is used during the intraoperative period. Peng and Zhang illustrate that 0.5 μg/kg/h dexmedetomidine does not prolong extubation time, while one μg/kg/h may prolong extubation time (27). Furthermore, Al-Zaben et al. discussed the effect of dexmedetomidine vs. saline on recovery time regarding respiratory recovery time, extubation time, spontaneous eye-opening time, spontaneous arm or leg motion time, and the time of discharge to the PACU and concluded that dexmedetomidine did not prolong recovery time (28).
Other investigations failed to find evidence on the longer emergence or discharge time (29-31). This inconsistency might have been caused by different doses, variable administration route, or timing of the dexmedetomidine and other opioids (19).
Although there was a tendency toward the lower incidence of these two postoperative complications in Group D, this study found no discernible difference in the incidence of coughs and PONV between the groups. The fact that both groups received ondansetron as an antiemetic and used less perioperative fentanyl can be blamed for the negligible difference in the incidence of PONV. For example, mouth dryness was more prevalent in Group D than in the control group in a trial study by Bajwa et al.; this medication protected against nausea, vomiting, and headache (32).
Similarly, Kim et al. found that dexmedetomidine 0.4 μg/kg/h offered no further benefit in reducing coughing (21).
Gupta et al. discovered that Group D revealed a considerably decreased incidence of postoperative nausea and vomiting (11.1% vs. 50%) (26).
On the other hand, most of our cases in the control group developed shivering postoperatively without a significant difference when compared to the caudal and dexmedetomidine groups at P = 0.306. Dexmedetomidine can be useful in controlling the shivering mechanism because it lowers the temperature threshold for shivering. Dexmedetomidine’s ability to reduce vasospasm and the effect of the agonist alpha-2 are two additional potential pathways in preventing shivering (33).
Regarding tachycardia, a significantly higher number of patients in the control group (66.7%) developed tachycardia when compared to the caudal and dexmedetomidine groups (20% and 22.2%, respectively, P < 0.001). This is reinforced by the fact that in animal research, dexmedetomidine inhibits epinephrine/halothane-induced ventricular tachycardia, and this putative antiarrhythmic function is associated with cerebral imidazoline receptors (34).
According to Li et al., after starting dexmedetomidine, the heart rate in group D decreased significantly and stayed significantly lower throughout the surgical procedure (P < 0.0001 for all groups) (18).
Unfortunately, there were several limitations in this study. The sevoflurane concentration was altered, precisely as in our trial, to keep the hemodynamic variables at their 20% baseline value. Accordingly, we could not evaluate the hemodynamic effects of dexmedetomidine single bolus dosages. Moreover, we did not compare the effects of various dexmedetomidine doses because we only employed one dose.
Furthermore, the study sample size is modest. We did not account for other issues while computing the sample size and only considered the decrease in MOPS. Despite these limitations, the study’s main strength is comparing the frequency and intensity of agitation using low dosages of dexmedetomidine not prolonging anesthetic recovery.
We can conclude that emergence benefits gained from the caudal block can be obtained by IV dexmedetomidine; however, it has less analgesic efficacy in pediatric patients undergoing hypospadias repair surgery without remarkable complications. Further clinical investigations are recommended to validate our findings.