1. Background
Hemorrhoids, the most common disease in and around the anus, are swollen veins similar to varicose veins in the anus and lower rectum. The clinical symptoms of hemorrhoids include pain, bleeding, itching, and lumps near the anus. Hemorrhoids are classified under grades I-IV according to their size. Grade II or higher grades of hemorrhoids require surgery because they do not respond to pharmacotherapy. Hemorrhoidectomy is considered a very unpleasant treatment for patients because it is associated with apparent postoperative pain, as pain relief in 40% of patients undergoing a hemorrhoidectomy requires the administration of narcotics (1).
There are various methods for post-hemorrhoidectomy pain management, the most common of which is the administration of injectable narcotics, usually combined with other drugs to increase their analgesic properties (2). Preemptive analgesia throughout the perioperative period with acetaminophen, gabapentin, ketamine, or dexamethasone is also recommended (3). Other methods, such as bilateral pudendal nerve block (4) and acupuncture (5), can also effectively relieve post-hemorrhoidectomy pain.
Ketorolac is an injectable non-steroidal anti-inflammatory drug (NSAID) that can be used alone or in combination with other drugs to control postoperative pain. Although there are reports concerning the topical administration of this drug to the surgical site for pain relief in minor to medium surgeries (6), few studies have addressed its topical administration in patients undergoing hemorrhoidectomy (7). However, this method has been employed in some cases of outpatient hemorrhoidectomies (8), and it is commonly used topically in clinical cases for post-hemorrhoidectomy pain management.
Hashemi et al.’s study investigated the effect of the type of anesthesia on pain control after hemorrhoidectomy. The first, second, and third surgery groups underwent general anesthesia, spinal anesthesia, and local anesthesia. In the aforementioned study, the third group that received local anesthesia not only experienced less pain after surgery but also complications, such as headache, hypotension, urinary retention, and respiratory complications, were also less frequent in this group (9). Akhtar et al. investigated the use of topical ketorolac in the treatment of pain after minor to moderate surgical procedures. One infiltration group with Marcaine and one infiltration group with ketorolac were performed at the operation site. The results showed better pain control in the group receiving Marcaine (6). A study showed that ketorolac buccal infiltration could reduce the postoperative pain experienced by patients requiring endodontic treatment diagnosed with symptomatic irreversible pulpitis (10).
2. Objectives
Using analgesics with less risk in reducing pain after surgery has always been one of the challenges for experts. Finding useful solutions requires clinical research. Therefore, this study aimed to investigate the effects of topical ketorolac on post-hemorrhoidectomy pain management.
3. Methods
This study was approved by the Ethics in Research Committee of Rafsanjan University of Medical Sciences, Rafsanjan, Kerman, Iran (IR.RUMS.REC.1399.242) and registered on the Iranian Registry of Clinical Trials (IRCT20210922052556N1). The statistical population consisted of patients with grade-II hemorrhoids who were admitted to Ali ibn Abi-Talib hospital of Rafsanjan for hemorrhoidectomy within 2020 - 2021. The inclusion criteria were an age range of 15 - 55 years, the American Society of Anesthesiologists I or II, non-affliction with coagulopathy, no history of gastrointestinal bleeding or peptic ulcer, no drug dependence, and no history of diabetes and uncontrolled hypertension. The exclusion criteria were withdrawal from the study and complications during surgery. The sample size was calculated based on α = 0.05, β = 0.20, σ1 = 1.30, σ2 = 1.33, K = 1, and Δ = 1, at 28 subjects in each group.
After obtaining informed consent from the participants, they were randomly assigned to three groups of 28 by drawing lots. The first, second, and third participants were assigned to the control, topical, and intramuscular groups, respectively. This process continued until the end of the study. An anesthesia resident taught the participants how to use the Numerical Pain Rating Scale (NPRS), one of the most reputable tools for measuring pain intensity, before the surgery. This tool measures pain intensity on an 11-point Likert scale from 0 (no pain) to 10 (the most severe pain). The participant chooses one of the numbers from 0 to 10 to describe pain intensity, and higher scores indicate greater pain severity (11).
All participants underwent surgery under general anesthesia. For this purpose, after administering 500 mL of normal saline, a mixture of 2 mg midazolam, 2 mL sufentanil, 2 mg/kg propofol, and 20 mg atracurium was injected. A laryngeal mask was also used for airway management in the patients. No medications were prescribed to control pain during the operation except in the subsequent cases. At the end of the surgery, the following interventions were performed for pain management:
Topical: 4 mL 0.5% Marcaine + 1 mL ketorolac at the surgical site
Intramuscular: 4 mL 0.5% Marcaine at the surgical site + 1 mL ketorolac intramuscularly
Control: 4 mL 0.5% Marcaine at the surgical site
It is noteworthy that the surgical procedure was performed by a single surgeon for all participants. Pain intensity was measured using the NPRS during the recovery (1 hour after surgery) and 6, 12, and 24 hours later. Pethidine was prescribed to the participants who reported a score of 7 or higher for pain intensity. The statistical analyst was blinded to group allocation.
The obtained data were statistically analyzed in SPSS software (version 21). The normal distribution of the data was evaluated using the Shapiro-Wilk test, and the results confirmed the normal distribution of the collected data (P = 0.07). Box’s M and Levene’s tests were also employed to assess the equality of covariance matrices and the equality of variances of the studied groups, respectively. The studied groups were compared for the mean pain intensity at different times using two-way repeated measures analysis of variance (ANOVA). In addition, the main effects of treatment type, pain intensity measurement time, and interaction effects of treatment type and the times of pain measurement were also evaluated. The significance level for the tests was set to 0.05.
4. Results
In this study, 39 (46.4%) and 45 (53.6%) patients were female and male, respectively. There was no significant difference between the three groups regarding age (P = 0.866). There was no significant difference between the three groups in the mean age and body mass index (P = 0.0457; Table 1).
| Variables | Topical (n = 28) | Intramuscular (n = 28) | Control (n = 28) | P-Valueb |
|---|---|---|---|---|
| Age, y | 29.89 ± 8.80 | 39.32 ± 8.46 | 30.93 ± 8.33 | 0.548 * |
| Body mass index, kg/m² | 26.9 ± 3.6 | 26.3 ± 2.4 | 25.9 ± 1.9 | 0.457 |
| Gender | 0.866 ** | |||
| Female | 14 (50.0) | 13 (46.4) | 12 (42.9) | |
| Male | 14 (50.0) | 15 (53.6) | 16 (57.1) |
a Values are expressed as mean ± SD or No. (%).
b*One-way analysis of variance **Chi-square test P < 0.05.
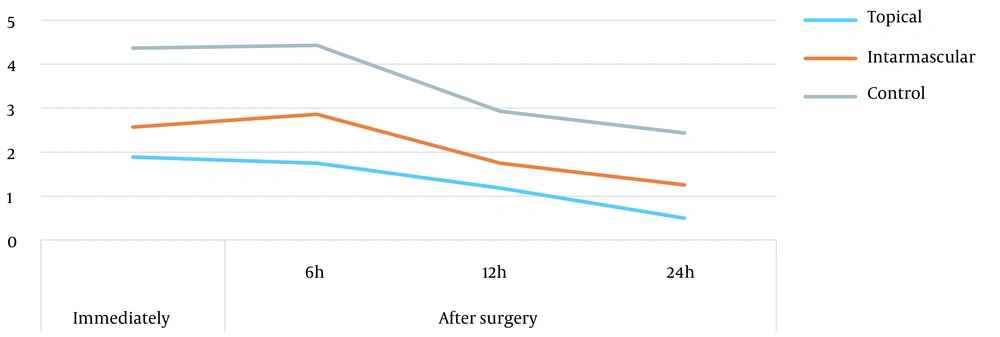
The mean scores of pain severity decreased over time in all three groups. The mean scores of pain intensity in all four measurement times in the control were higher than those of the topical group, and the mean scores of pain intensity in all four measurement times in the topical group were higher than those of the intramuscular group (Table 2).
| Measurement Time | Topical (n = 28) | Intramuscular (n = 28) | Control (n = 28) | |
|---|---|---|---|---|
| After surgery | Immediately | 1.89 ± 1.73 | 2.57 ± 1.97 | 4.36 ± 2.23 |
| 6 hours | 1.75 ± 1.04 | 2.86 ± 1.24 | 4.43 ± 2.20 | |
| 12 hours | 1.18 ± 0.82 | 1.75 ± 0.89 | 2.93 ± 1.02 | |
| 24 hours | 0.50 ± 0.69 | 1.25 ± 0.59 | 2.43 ± 1.14 |
a Values are expressed as mean ± SD.
One hour after the surgery, eight, four, and three participants in control, intramuscular, and topical groups, respectively, were treated with pethidine due to high pain intensity. In addition, only those in the control group received pethidine 6 hours after the surgery for pain relief. None of the participants needed pethidine for pain management 12 and 24 hours after the surgery (Table 3).
Two-way repeated measures ANOVA was used to study the trends of changes in the scores for pain intensity in the three groups. Considering the significance of the effect of time, pain intensity in the participants showed a decreasing trend in general up to 24 hours after the surgery (P < 0.001). The significance of the intervention effect also indicated significant differences between the three groups in pain intensity, as pain intensity in the topical group was lower than in the intramuscular group and lower in the intramuscular group than in the control group (P < 0.001). The results demonstrated that the interactive effects of time and intervention were not statistically significant, indicating no significant differences between the three groups in terms of changes in pain intensity over time (P = 0.104; Table 4).
| Time of Pethidine Administration | Group | Total | P-Value | ||
|---|---|---|---|---|---|
| Topical | Intramuscular | Control | |||
| Immediately after surgery | 0.18 | ||||
| Yes | 3 (10.7) | 4 (14.3) | 8 (28.5) | 15 (17.8) | |
| No | 25 (89.3) | 24 (85.7) | 20 (71.5) | 69 (82.2) | |
| Total | 28 (100) | 28 (100) | 28 (100) | 84 (100) | |
| 6 hours after surgery | - | ||||
| Yes | 0 | 0 | 3 (10.7) | 3 (3.6) | |
| No | 28 (100) | 28 (100) | 25 (89.3) | 81 (96.4) | |
| Total | 28 (100) | 28 (100) | 28 (100) | 84 (100) | |
a Values are expressed as No. (%).
| Source of Variation | Sum of Squares | df | Mean Squares | F | P-Value |
|---|---|---|---|---|---|
| Time | 136.272 | 1 | 136.572 | 119.282 | < 0.001 |
| Intervention + Time | 5.337 | 2 | 2.668 | 2.331 | 0.104 |
| Intervention | 280.292 | 2 | 140.146 | 24.122 | < 0.001 |
| Error | 470.598 | 81 | 5.810 |
Considering the significance of the intervention effect and the presence of three groups in the study, Tukey’s test for post-hoc analysis was employed for pairwise comparisons between the groups. The results showed that the mean score of pain intensity in the intramuscular group was higher than in the topical group (P = 0.009). The mean score of pain intensity in the control group was higher than in other groups (P < 0.001; Table 5).
| I | J | Mean Difference (I-J) | SE | P-Value |
|---|---|---|---|---|
| Topical | Intramuscular | -0.773* | 0.288 | 0.009 |
| Control | -1.978* | 0.261 | < 0.0001 | |
| Intramuscular | Control | -1.205* | 0.241 | < 0.0001 |
Among all measurement times, the highest and the lowest mean scores of pain intensity were those of the control and topical groups, respectively. The score of pain intensity decreased over time in all groups; however, the trends of changes in pain intensity were identical in all three groups (in the shape of three parallel lines) (Figure 1).
5. Discussion
This study addressed the effects of topical ketorolac on post-hemorrhoidectomy pain management. The three treatment methods investigated in this study were the topical administration of Marcaine and ketorolac, intramuscular administration of Marcaine and ketorolac, and topical administration of Marcaine. The results showed the effectiveness of all these three interventions in relieving post-hemorrhoidectomy pain. However, pain intensity one hour after the surgery was significantly lower in the topical administration of the Marcaine and ketorolac group. In addition, the participants in this group needed lower doses of opioids than those in other groups within the first 24 hours after the surgery. This finding is consistent with the findings of Dehbozorgi, who studied the prophylactic effect of the intravenous administration of ketorolac on post-hemorrhoidectomy pain relief (12).
Vatankhah and Melekshoar reported that the intravenous injection of 30 mg ketorolac was more effective than the administration of 400 mg ibuprofen in pain relief among patients undergoing upper extremity orthopedic surgery, especially within the first 6 hours after surgery (13). The findings of Ebtehaj also indicated that the analgesic effect of the intramuscular administration of ketorolac was similar to that of the intramuscular injection of 75 mg pethidine in post-cesarean section pain management while causing fewer complications (14). Nevertheless, Zangoue et al. showed no significant difference between patients treated with ketorolac and pethidine in post-cesarean section pain intensity (15). Considering the fewer complications of ketorolac and the limitations of pethidine administration, ketorolac can be a suitable alternative to pethidine for post-cesarean section pain management. The results of two studies on patients undergoing inguinal hernia surgery (16) and lower limb orthopedic surgery (17) confirmed the effectiveness of ketorolac, compared to acetaminophen.
Abdoli et al. concluded that ketorolac could be a good alternative to morphine for pain control in patients with spinal traumas (18). In another study by McDonald et al., it was shown that the addition of ketorolac to the postoperative drug regimen reduced the need for opioids after open reduction and internal fixation of ankle fractures in the early postoperative period and showed mixed and minor effects on pain reduction (19). The findings of Ong and Tan indicated that the preoperative intravenous administration of 30 mg ketorolac was more effective than the injection of 50 mg tramadol in preventing postoperative dental pain (20). Another study compared the effectiveness of ibuprofen and ketorolac in reducing renal colic pain, and the results showed that ibuprofen caused analgesic effects faster than ketorolac. In addition, the complete pain relief rate was higher in patients treated with ibuprofen than in those who received ketorolac (21).
The results of another study demonstrated the better analgesic effects of intravenous ketorolac than nebulized fentanyl in patients with renal colic pain (22). The evidence indicated that injectable ketorolac and morphine produced almost the same analgesic effects in patients with sickle cell disease; nevertheless, ketorolac caused fewer complications (23). However, Tirupathi et al. conducted a review study and concluded that further clinical trials were needed to prove the effectiveness of ketorolac in pain control (24). By contrast, Isiordia et al. showed that the administration of 30 mg ketorolac, compared to 1 - 10 mg parecoxib, after the surgical removal of third molars resulted in better analgesic effects and higher patient satisfaction (25).
Numerous studies have also investigated the effectiveness of ketorolac in children and adolescents. Lynn et al. studied the efficacy of postoperative administration of ketorolac in infants aged 6 - 18 months and reported no adverse effects on surgical site drainage, oxygen saturation, and renal or liver functions (26). Due to the lack of reliable information and evidence about primary consequences, the efficacy and safety of ketorolac in reducing postoperative pain in children are still unknown (27). In addition, there are no randomized and controlled clinical trials with a placebo addressing the effects of ketorolac on infants. However, most published reports have demonstrated the efficacy and safety of ketorolac in appropriately selected infants (28). Although the intravenous administration of ketorolac to children for postoperative pain relief is not approved in many countries, it is commonly employed in clinical cases (28).
The analgesic effects of ketorolac have also been studied in animal models. In a study, ketorolac not only decreased pain but also significantly reduced the tolerance of the analgesic effects of morphine and the symptoms of withdrawal syndrome caused by the administration of naloxone in rats (29). Guidelines for postoperative pain management suggest a combination of multidimensional/multi-mechanism approaches to achieve better postoperative outcomes. A multimodal approach means the simultaneous use of two or more analgesics (usually a combination of opioid and non-opioid ones) with different mechanisms of action. A multimodal approach not only minimizes the need for the administration of opioids but also results in greater pain relief when compared to using a single treatment. As a result, it reduces the side effects associated with narcotic painkillers. However, not all methods might be effective for all patients, and not all analgesics might be appropriate for outpatient surgeries or urgent surgical procedures (30).
The NSAIDs play a major role in pain management in various clinical conditions, such as headache, menstrual disorders, postoperative pain, spinal and soft tissue pain, rheumatoid arthritis, and osteoarthritis, with cyclooxygenase enzyme blockade (31). There is very low to moderate certainty evidence to prove the efficacy and safety of ketorolac as a treatment for postoperative pain relief. Although available evidence suggests that the intravenous administration of ketorolac might provide remarkable postoperative pain relief for most patients, future studies might affect this estimation. The side effects of ketorolac occur at a slightly higher rate than placebo and other NSAIDs. There is insufficient information available to assess whether the intravenous administration of ketorolac results in a different rate of gastrointestinal or surgical site bleeding, renal dysfunction, or cardiovascular events compared to other NSAIDs. Insufficient research has been conducted on old patients undergoing cardiovascular surgery who might be at risk of adverse events (32).
This study, similar to any other study, had some limitations. All three investigated groups (intervention cases) in the present study received topical analgesic drug treatment. It would have been better to have a group as a control in the study and compare the pain intensity to that group. However, this measure was abandoned due to the need to control the patients’ pain after the operation and respect the patients’ rights.
5.1. Conclusions
The topical administration of ketorolac and Marcaine was more effective than Marcaine used alone for relieving pain in patients undergoing hemorrhoidectomy. This study investigated the topical administration of ketorolac, which causes fewer complications than its intravenous administration.