1. Background
Pediatric gastrointestinal (GI) endoscopy has evolved over the last 40 years with increasing diagnostic and therapeutic uses (1). This procedure is generally more annoying for pediatric patients than the symptoms that led to endoscopy (2). Despite many applications, the pain, discomfort, and anxiety caused by this procedure can make the conditions challenging for the patients and the appliers, which makes GI endoscopy a procedure that usually requires sedation (3, 4). Adequate sedation is crucial in pediatric GI endoscopy, as insufficient sedation can lead to severe complications (5, 6). Anesthesiologists use various sedative agents to provide appropriate sedation during upper GI endoscopy in children. However, limited therapeutic choices, such as hypnotics, opioids, and benzodiazepines, can be safely administered to pediatric patients (7-9). In a survey of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) members, pediatric gastroenterologists could not achieve a consensus protocol for optimum sedation for gastrointestinal endoscopy (10).
Ketamine is an N-methyl-D-aspartate (NMDA) receptor antagonist commonly used in pediatric anesthesia with pleiotropic effects, such as pain relief, amnesia, and sedation (11-13). Ketamine is a commonly used sedative for pediatric upper gastrointestinal endoscopy due to its rapid onset of action and short half-life. Studies showed anxiety, irritability, and nausea to more severe adverse effects such as dyspnea and laryngospasm. Ketamine must not be administered in infants younger than 3 months or patients with unstable airways, cardiac diseases, central nervous system diseases, mental disorders, porphyria, or thyroid diseases (14-16). It has been shown that using lower doses of ketamine in combination with other sedatives, such as benzodiazepines, can effectively reduce the adverse effects of this drug (17). The combination of ketamine and propofol for sedation and pain relief has been widely studied. Sedation with propofol and ketamine provides various advantages over monotherapy (18). For decades, lidocaine has been used clinically for local anesthesia and treating cardiac arrhythmias. The intravenous (IV) administration of lidocaine has shown favorable effects on postoperative pain (19-22). The anti-inflammatory effects of IV lidocaine have been assessed in various clinical investigations on adults (23, 24). Analgesic and anti-inflammatory effects of IV lidocaine help patients recover after surgery with less discomfort, improved gastrointestinal symptoms (such as nausea and vomiting), and shorter length of hospitalization (25, 26).
Studies have indicated favorable effects with fewer adverse effects in favor of combination therapy for sedation during pediatric endoscopy. Ketamine is often used for sedation during this procedure (27), and IV lidocaine is still being studied as an adjuvant anesthetic for sedation and analgesia (28, 29).
2. Objectives
So far, no previous study has examined the effect of IV lidocaine and ketamine on the sedation and hemodynamic parameters of patients undergoing pediatric endoscopy. Therefore, we aimed to evaluate the effect of IV lidocaine as an adjunct to IV ketamine on the hemodynamic parameters of patients undergoing pediatric GI endoscopy, as well as investigate the effect of this combination on endoscopist satisfaction and recovery room length of stay as outcomes.
3. Methods
3.1. Study Design and Population
This triple-blind, randomized, controlled clinical trial was conducted in Imam Hossein Hospital, Isfahan, Iran (2021). Isfahan University of Medical Sciences funded this study. The study protocol was approved by the Ethics Committee of Isfahan University of Medical Sciences (code: IR.MUI.MED.REC.1400.625); in addition, it was registered on the Iranian Registry of Clinical Trials website (code: IRCT20211108053008N1)
Eligible patients were pediatric upper GI endoscopy candidates aged 1 to 6 years with the consent of parents or legal guardians for participation in the study. Patients with coryzal symptoms within the last 2 weeks, a history of reactive airway disease, asthma or other respiratory diseases, epilepsy, increased Intracranial pressure (ICP, cardiac diseases, neurological diseases, mental health disorders, porphyria, or thyroid disorders were excluded from the study.
3.2. Randomization and Blinding
Using SPSS version 22 (SPSS Inc, Chicago, IL, USA), patients were randomly assigned in a 1:1 ratio to one of the 2 groups of lidocaine + ketamine or ketamine alone. Masked blocks of 4 prepared by a non-blind statistician were allocated to trained nurses to perform interventions. The data collector, patient, and authors were unaware of the grouping.
3.3. Interventions
The lidocaine + ketamine group received 1.0 mg/kg of lidocaine and 1.0 mg/kg of ketamine intravenously, and the ketamine group received 1.0 mg/kg of IV ketamine and normal saline as a placebo 2 minutes before transferring to the operating room. Patients in both groups were sedated with 1.0 mg/kg of propofol, 0.1 mg/kg of midazolam, and 2.0 ug/kg of fentanyl for the procedure. During the study, patients were closely monitored regarding their hemodynamic status and adverse effects.
3.4. Data Collection
All data were collected by 1 trained nurse. Demographic information (age, weight, and gender) was collected before initiating the intervention. Patients’ hemodynamic status (heart rate, mean arterial pressure [MAP], respiratory rate, and oxygen saturation) was recorded 1 minute before the intervention and every 5 minutes afterward (10, 11, 13, 22, 30, 31). Furthermore, the endoscopist satisfaction based on a Likert scale and the duration of recovery room stay were recorded.
3.5. Statistical Analysis
The collected data were analyzed using SPSS version 22. Quantitative data were shown as mean ± SD, and qualitative data were presented as frequency (percentages). For inferential analysis, data were analyzed using the chi-square test, t-test, and 1-way analysis of variance (ANOVA). P-values less than 0.05 were considered statistically significant.
4. Results
A total of 120 patients were enrolled. The mean (SD) of patients was 3.4 (1.4) in the ketamine + lidocaine group and 3.4 (1.7) in the ketamine group. Sixty participants were enrolled in each group. Demographic findings indicated in Table 1 showed no statistically significant differences between the 2 groups regarding sex (P = 0.58), age (P = 0.77), and weight (P = 0.89).
| Variables | Ketamine | Ketamine + Lidocaine | P-Value |
|---|---|---|---|
| Age, y | 3.4 ± 1.7 | 3.4 ± 1.4 | 0.77* |
| Weight, kg | 13.5 ± 3.1 | 13.6 ± 3.4 | 0.89* |
| Sex | 0.58** | ||
| Male | 27 (45.0) | 30 (50.0) | |
| Female | 33 (55.0) | 30 (50.0) |
a Values are expressed as mean ± SD or No. (%). * P-values based on the 1-way analysis of variance. ** A P-value based on the chi-square test.
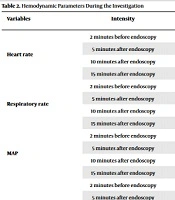
Table 2 indicates the hemodynamic parameters of patients during the investigation. No significant differences were found regarding heart rate (P = 0.99), respiratory rate (P = 0.70), MAP (P = 0.98), and oxygen saturation (P = 0.23) at baseline. Also, it was seen that 5 minutes after initiating the endoscopy, the mean difference in heart rate (P = 0.71), respiratory rate (P = 0.47), MAP (P = 0.82), and oxygen saturation (P = 0.74) were not significant between the 2 groups. There were no significant differences between the 2 groups regarding the means of heart rate (P = 0.88), respiratory rate (P = 0.36), MAP (P = 0.85), and oxygen saturation (P = 0.57) 10 minutes after endoscopy. No significant differences were found regarding the means of heart rate (P = 0.96), respiratory rate (P = 0.53), MAP (P = 0.23), and oxygen saturation (P = 0.40) 15 minutes after endoscopy between the 2 groups.
| Variables | Intensity | Ketamine, Mean ± SD | Ketamine + Lidocaine, Mean ± SD | P-Value a |
|---|---|---|---|---|
| Heart rate | 2 minutes before endoscopy | 134.43 ± 16.93 | 134.40 ± 12.67 | 0.99 |
| 5 minutes after endoscopy | 127.53 ± 14.59 | 128.45 ± 12.96 | 0.71 | |
| 10 minutes after endoscopy | 126.47 ± 14.40 | 126.82 ± 12.85 | 0.88 | |
| 15 minutes after endoscopy | 126.08 ± 14.64 | 125.98 ± 12.74 | 0.96 | |
| Respiratory rate | 2 minutes before endoscopy | 14.75 ± 2.52 | 14.92 ± 2.29 | 0.70 |
| 5 minutes after endoscopy | 12.62 ± 2.26 | 12.90 ± 2.09 | 0.47 | |
| 10 minutes after endoscopy | 11.82 ± 2.08 | 12.15 ± 1.92 | 0.36 | |
| 15 minutes after endoscopy | 11.37 ± 1.81 | 11.58 ± 1.96 | 0.53 | |
| MAP | 2 minutes before endoscopy | 63.30 ± 8.35 | 63.33 ± 7.76 | 0.98 |
| 5 minutes after endoscopy | 61.82 ± 7.74 | 62.13 ± 7.84 | 0.82 | |
| 10 minutes after endoscopy | 61.47 ± 7.70 | 61.20 ± 7.69 | 0.85 | |
| 15 minutes after endoscopy | 61.10 ± 7.56 | 61.07 ± 7.62 | 0.98 | |
| Oxygen saturation | 2 minutes before endoscopy | 97.55 ± 2.11 | 98.00 ± 2.05 | 0.23 |
| 5 minutes after endoscopy | 98.02 ± 1.927 | 98.13 ± 1.926 | 0.74 | |
| 10 minutes after endoscopy | 98.13 ± 1.80 | 98.32 ± 1.73 | 0.57 | |
| 15 minutes after endoscopy | 98.33 ± 1.81 | 98.60 ± 1.67 | 0.40 |
Abbreviation: MAP, mean arterial pressure.
a P-values based on the t-test.
Table 3 indicates the endoscopist satisfaction scores and patient recovery time. Endoscopist satisfaction scores (P = 0.83) and patient recovery time (P = 0.79) were not significantly different between the 2 groups.
| Variables | Ketamine, Mean ± SD | Ketamine + Lidocaine, Mean ± SD | P-Value a |
|---|---|---|---|
| Endoscopist satisfaction (Likert criteria) | 4.77 ± 0.42 | 4.75 ± 0.43 | 0.83 |
| Patient recovery time, min | 31.08 ± 11.72 | 31.65 ± 11.65 | 0.79 |
a P-values based on the t-test
5. Discussion
Proper sedation in children is essential for various procedures (4, 5). Multiple studies have investigated the effects of different medications for pediatric procedural sedation to enhance favorable outcomes and minimize adverse effects (7-9). Although many drug choices are available for sedation in pediatric GI endoscopy, ketamine is more commonly used due to its effectiveness and fewer adverse effects (11-13). Studies have shown that using ketamine in pediatric sedation may be associated with some complications, such as hemodynamic instability, hallucinations, and excessive sedation. Reducing the dose of ketamine can be beneficial in minimizing these complications. Therefore, many studies have recommended combination therapy due to improved outcomes and fewer complications, such as respiratory adverse effects (17, 18, 20). Mortero et al. have shown that adding low-dose ketamine to propofol reduced respiratory complications (32).
In recent years, IV lidocaine has been studied as an adjuvant in pediatric sedation, showing beneficial effects in combination with other sedatives, such as improved hemodynamic stability (25, 26, 33). Forster et al. evaluated 40 adults and showed that IV lidocaine in combination with ketamine and propofol for colonoscopy reduced the required doses of propofol (34). Although preoperative administration of lidocaine is currently recommended in adult patients, the current recommendations regarding the use of this drug in children are still contradictory (35). In this study, we investigated the effect of adding IV lidocaine to ketamine on the hemodynamic status, endoscopist satisfaction, and patient recovery time in pediatric patients undergoing GI endoscopy. Our study showed that adding lidocaine to ketamine did not significantly affect MAP, heart rate, respiratory rate, and oxygen saturation. Furthermore, this combination did not significantly affect the endoscopist satisfaction and patient recovery time.
Intravenous ketamine can be associated with increased blood pressure and heart rate (36). In a case-control study by Fang et al., half of the patients were sedated with ketamine and midazolam, and the other half received 2 mg/kg of IV lidocaine along with ketamine and midazolam. Unlike the case group, intraoperative and postoperative systolic blood pressure, heart rate, and respiratory rate showed an increasing trend in the control subjects (37). The difference between these results and our investigation can be attributed to the different doses of lidocaine and ketamine used in the 2 studies.
Studies have shown that IV lidocaine infusion during pediatric laparotomy appendectomy reduces the required doses of opioids during surgery (38). Lidocaine infusion has also been shown to decrease hospitalization duration and volatile anesthetic requirement (39-41). Another study investigated the effect of adding lidocaine to propofol in colonoscopy patients with a mean age of approximately 7 years. In addition to propofol, patients in the intervention group received 1.5 mg/kg of IV lidocaine along with infusion. Patients in the control group also underwent IV lidocaine induction in addition to propofol. The propofol requirement and recovery time were significantly lower in the intervention group than in the control group. There was no significant difference between the 2 groups regarding complications such as hypotension, bradycardia, and hypoxia (29). Conflicting results of these studies and our study can be attributed to intraoperative lidocaine infusion in these studies, different anesthesia methods, and the younger age of our participants.
Some previous studies have not shown results in favor of pediatric sedation with IV lidocaine. Depue et al. showed that the combined treatment of 0.25 to 0.5 mg/kg of preoperative IV lidocaine with propofol infusion did not significantly affect the pain and distress of 2 - 7 years old patients and the distress of their parents and physicians during the procedure (42). In another study of children aged 3 - 17, lidocaine infusion during laparoscopic appendectomy did not improve circulatory and respiratory alterations during pneumoperitoneum (35).
The present study had some limitations. This investigation was a single-center study with a small sample size due to the limited budget. In such studies, it is better to monitor the patients regarding the onset of anesthesia duration and anesthesia requirements during the procedure and follow them up until the end of the hospitalization regarding complications. Despite these weaknesses, our study also had strengths. Unlike most previous studies, our study examined patients under 6 years old. On the other hand, our study investigated the effects of preoperative IV lidocaine (not infusion) with a minimum dosage and its infusion, which can be considered both a weakness and a strength.
5.1. Conclusions
Adding low-dose IV lidocaine to ketamine in patients undergoing GI endoscopy did not affect the hemodynamic status or endoscopist satisfaction and recovery time as outcomes.