1. Background
Surgeries are sometimes associated with an altered cognitive function reflected by impairment of attention, perception, language comprehension, social integration, memory, and information processing, known as postoperative cognitive dysfunction (POCD) (1), affecting patients in all age groups (2). Since women who have had cesarean section (CS) have been shown to have poorer relationship outcomes with their newborns, POCD may also affect the infant-mother relationship (3). Breastfeeding can also be affected because general anesthesia (GA) may cause increased sleepiness of the baby, encouraging the use of supplementary bottles to give the fatigued mother a rest (4).
Although the mechanisms leading to cognitive disturbances are still unknown, studies attributed it to the immune response to surgery. The inflammatory response could lead to the development of POCD through inducing the secretion of cortisol, cytokines, and other inflammatory mediators (5, 6). It is noteworthy that age, type of surgery, duration of anesthesia, education, the use of certain anesthetics, number of previous surgeries, postoperative infections, and respiratory complications seem to increase the risk of POCD (7, 8). Indeed, preeclampsia is the most significant cause of POCD, whereas preeclamptic women complain of POCD more often compared to women who have had uncomplicated deliveries (9).
It takes time for psychomotor functions to return to the preoperative levels after the anesthesia is eliminated (10). It has been demonstrated that after being exposed to anesthesia, cognitive and psychomotor functions are impaired for 10 to 12 hours, and these symptoms may even occur with the smallest anesthetic (11, 12). Rapid recovery of patients who have been under GA and their returning to their pre-anesthesia condition mentally are major goals for anaesthesiologists (13, 14). Thence, evaluation of the cognitive behavior preoperatively is essential since mild cognitive impairment may be worsened following a CS (15).
In addition, the choice of an anesthetic method for CS depends on the reason for the procedure, its degree of urgency, and on the preference of both the patient and anesthesiologist. There is no anesthetic method ideal for CS. The anesthesiologists must choose a method they believe is the safest and most comfortable for the mother and the newborn, and ensures optimal operating conditions for the surgery (16). Some studies suggest that GA and insufficient postoperative pain control play a role in the development of early POCD (17, 18). Yet, most of the studies about POCD have methodological problems as pointed out in a systematic review of more than 40 studies (19).
2. Objectives
This comparative observational study aimed to assess the incidence of early POCD after elective and emergent CS under GA.
3. Methods
3.1. Ethical Statement
The study was carried out at Beni-Suef University Hospital after approval from the Anesthesia Department and Local Ethics Committee of Faculty of Medicine, Beni-Suef University, Egypt. Written informed consent was obtained from all patients. All the methods were conducted in accordance with the relevant guidelines and regulations, as well as the declaration of Helsinki.
3.2. Sample Size
To calculate sample size, we compared early POCD occurrence between cases after elective and those after emergent CS under GA, as it was the primary outcome of the study. As reported in a previous publication (15), we assumed that emergent CS causes cognitive dysfunction in at least 10 patients. The minimum calculated sample size was 24 patients in each arm to reject the null hypothesis with 80% power at α = 0.05 level using Student’s t-test. We used G*Power software version 3.1.2. If the power increased to 90%, the sample was 25, and if the power increased to 95%, the sample was 27. So, we enrolled 29 patients in each group to account for the loss of follow-up.
3.3. Study Design
All patients had a full preoperative history taking and examination. A routine hematological and biochemical testing, along with electrocardiograms were performed. In the operating room, we considered standard continuous monitoring of electrocardiography, noninvasive arterial BP, end-tidal carbon dioxide (ETCO2), and pulse oximetry. All patients received lactate ringer's solution at approximately 3 - 5 mL/kg/hour perioperatively, and normothermia was maintained during the whole procedure. Anesthesia was induced by injecting 2.5 mg/kg propofol, 2 μg/kg fentanyl, and 0.5 mg/kg atracurium for muscle relaxation. Using 100% oxygen at a rate of 4 L/minute and sevoflurane 2%, patients were ventilated via face mask. We intubated the patients after 3 minutes using an appropriately sized cuffed oral tube lubricated with lidocaine jelly 2%. Also, sevoflurane 2% in 100% O2 was used to maintain anesthesia. Muscle relaxation continued by using atracurium 0.1 mg/kg every 20 minutes. To maintain ETCO2 between 35 - 40 mmHg, all patients were mechanically ventilated. We recorded the mean arterial blood pressure (MAP), heart rate (HR), and peripheral oxygen saturation (SpO2) immediately prior to induction of anesthesia and then every 5 minutes till the termination of anesthesia. Next, the patients were extubated on the recovery of adequate tidal volume and sufficient respiratory efforts and transferred to the recovery room for observation.
In addition, to evaluate cognitive function, neurocognitive mini mental state examination (MMSE) was used. We chose the MMSE because it is highly valid, easily applicable, and reliable. It consists of questions evaluating orientation to time, place, and person, short-term recall, attention, calculation, language, ability, and constructional ability (20). MMSE was performed one hour preoperatively, as well as 1, 6, and 24 hours postoperatively. The maximum score was 30 points, a decrease of ≥ 2 was considered as cognitive function decline, while a score < 23 was considered as cognitive impairment. Aldrete’s post-anesthesia recovery score was applied to each patient (21), and only score ≥ 7 was considered as the patient being awake, and MMSE was applied.
3.4. Eligibility Criteria
We included patients aged 20 - 40 years old having I-II ASA physical status. We excluded patients having preeclampsia or hypertension, ASA more than II, receiving sedatives as midazolam, and/or known or suspected neurological insult. Furthermore, we excluded patients if the time of surgery exceeded 60 minutes.
3.5. Assessment Parameters
The primary outcome was the occurrence of postoperative dysfunction, while secondary outcomes were blood loss and time of surgery. The amount of blood loss was determined by collecting the blood and rinsed fluid from the surgical field into the suction bottle by suctioning. A trained nurse who was not a part of the study made a visual assessment of blood-soaked gauge pieces that was consumed during surgery.
3.6. Statistical Analysis
The skewness and kurtosis tests were used for testing the normal distribution of continuous variables. Student’s t-test was used for continuous variables normally distributed, while Mann–Whitney U test was used for non-normal continuous variables. We used Fisher's exact test to compare the categorical variables. Paired t-test was applied for intragroup comparison of the mean values from preoperative to different time intervals for each group separately. Moreover, Student’s t-test (or Mann–Whitney U test, as appropriate) for intergroup comparison (i.e., elective versus emergent group) was conducted to compare mean values (calculated as follows: the mean of preoperative value - the mean of each time interval, i.e., 15, 30, and 45 minutes). Data were analyzed using the Statistical Package for the Social Sciences (SPSS) version 17 and RStudio software version 3.2.4. Results were considered significant if P < 0.05.
4. Results
4.1. Patients’ Characteristics
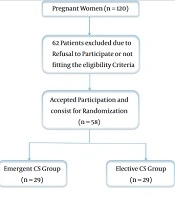
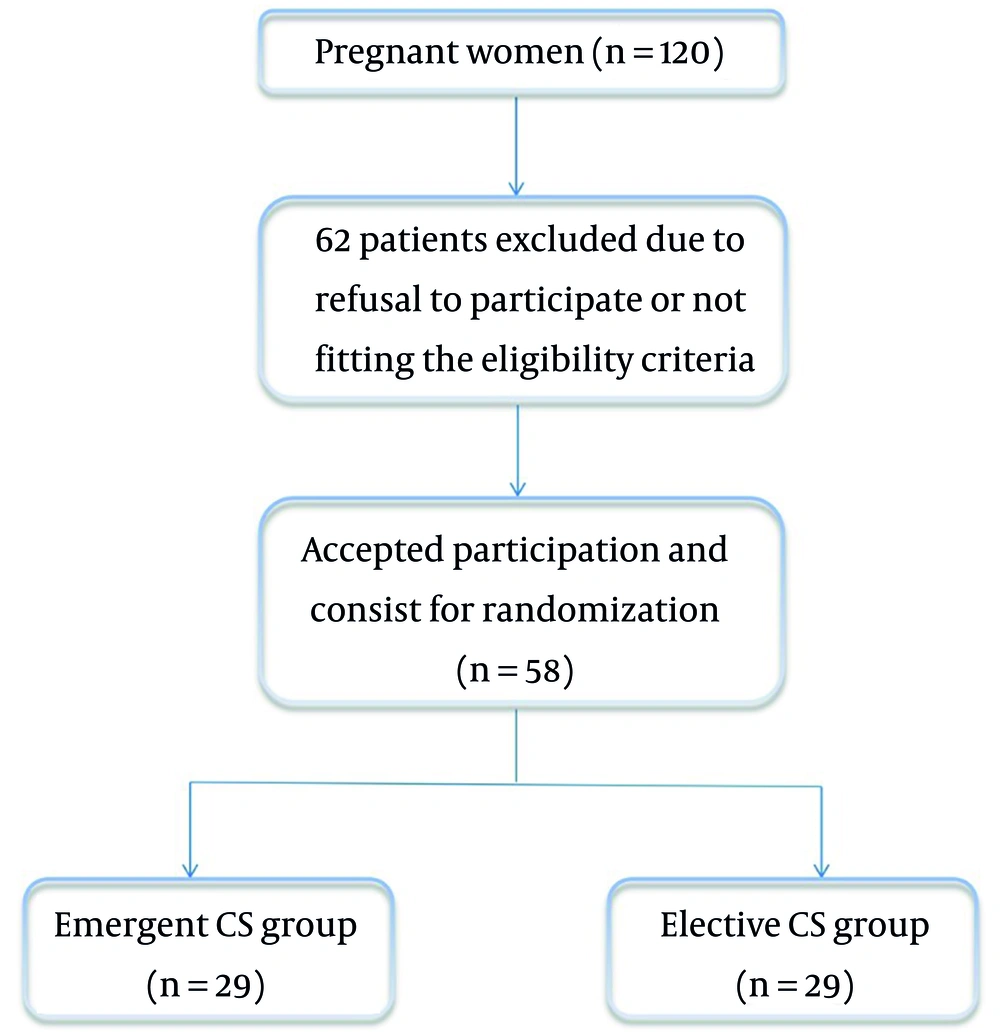
Out of 120 women, we enrolled 58 eligible women who underwent elective or emergent CS at Beni-Suef University Hospital (Figure 1). Patients’ age, weight, body mass index (BMI), and height at delivery were similar between the two groups (Table 1).
| Parameter | Elective (n = 29) | Emergent (n = 29) | P-Value |
|---|---|---|---|
| Age (y) | 30.21 ± 6.23 | 29.71 ± 6.22 | 0.765 |
| Weight (kg) | 77.18 ± 13.32 | 77.14 ± 14 | 0.992 |
| BMI (kg/m2) | 30.37 ± 4.61 | 30.42 ± 4.96 | 0.971 |
| Height (cm) | 159.32 ± 8.44 | 159.07 ± 8.64 | 0.913 |
| Duration of surgery (min) | 51.43 ± 6.78 | 50.18 ± 7.26 | 0.509 |
| Blood loss (mL) | 800 ± 99.07 | 817.86 ± 88.42 | 0.48 |
Characteristics of the Included Patients
4.2. Outcomes
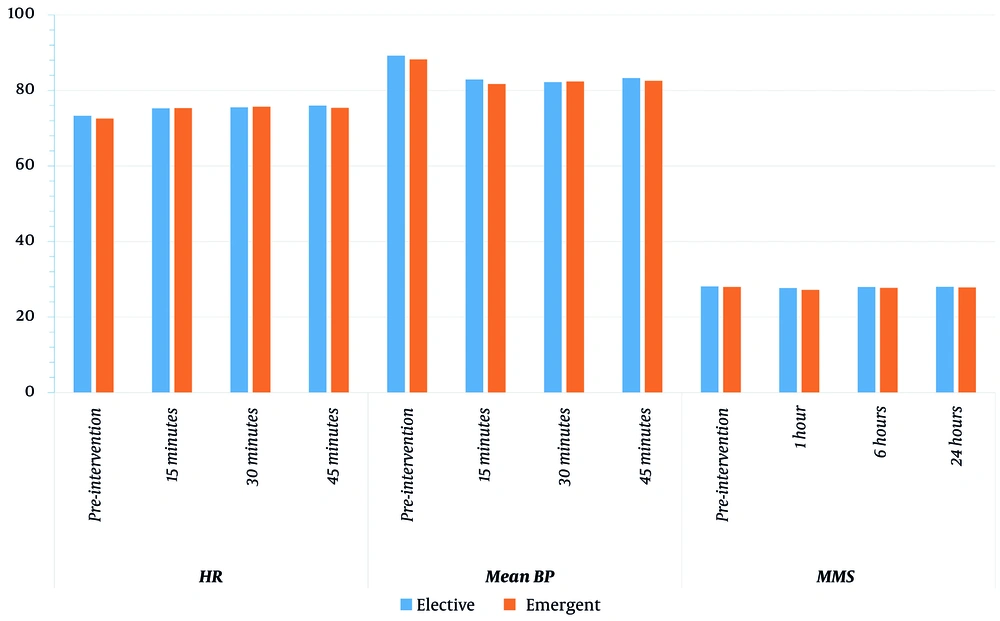
Regarding the intragroup comparison using paired t-test, we found a significantly lower MMSE at 1 hour (27.18 ± 1.74) as compared to preoperative MMSE (27.96 ± 1.35) in the emergent group, and MMSE tended to return to normal values faster in the elective than in the emergent group. Moreover, we found a significantly lower MBP and higher HR (at 15, 30, and 45 minutes) in both groups compared to preoperative values (Table 2 and Figure 2).
Differences between mean values of the study time intervals and the preoperative values These values were calculated as follows: the mean of preoperative value – the mean of each time interval (i.e., 15, 30, and 45 minutes). Abbreviations: HR, heart rate; BP, blood pressure; MMS, mini-mental state.
| Parameter | Elective (n = 29) | Emergent (n = 29) | P-Value b | P-Value c | |
|---|---|---|---|---|---|
| HR | Preoperative | 73.29 ± 7.87 | 72.57 ± 9.79 | 0.765 | - |
| 15 minutes | 75.25 ± 7.28 | 75.32 ± 9.55 | 0.975 | 0.004 / < 0.001 | |
| 30 minutes | 75.5 ± 7.52 | 75.68 ± 9.13 | 0.937 | 0.007 / < 0.001 | |
| 45 minutes | 75.96 ± 7.79 | 75.39 ± 8.53 | 0.794 | < 0.001 / < 0.001 | |
| Mean BP | Preoperative | 89.21 ± 4.61 | 88.21 ± 3.93 | 0.386 | - |
| 15 minutes | 82.89 ± 4.81 | 81.71 ± 3.9 | 0.318 | < 0.001 / < 0.001 | |
| 30 minutes | 82.21 ± 4.42 | 82.36 ± 3.87 | 0.898 | < 0.001 / < 0.001 | |
| 45 minutes | 83.25 ± 4.57 | 82.57 ± 3.63 | 0.541 | < 0.001 / < 0.001 | |
| MMS | Preoperative | 28.11 ± 1.52 | 27.96 ± 1.35 | 0.712 | - |
| 1 hour | 27.68 ± 1.76 | 27.18 ± 1.74 | 0.291 | 0.09 / 0.005 | |
| 3 (10.7) | 8 (28.57) | 0.177 | - | ||
| 6 hours | 27.93 ± 1.59 | 27.71 ± 1.63 | 0.62 | 0.326 / 0.129 | |
| 1 (3.57) | 3 (10.7) | 0.611 | - | ||
| 24 hours | 28 ± 1.49 | 27.82 ± 1.54 | 0.661 | 0.326 / 0.326 | |
| 1 (3.57) | 1 (3.57) | 1 | - | ||
The Mean Parameter Values for Each Group Across Different Time Intervals a
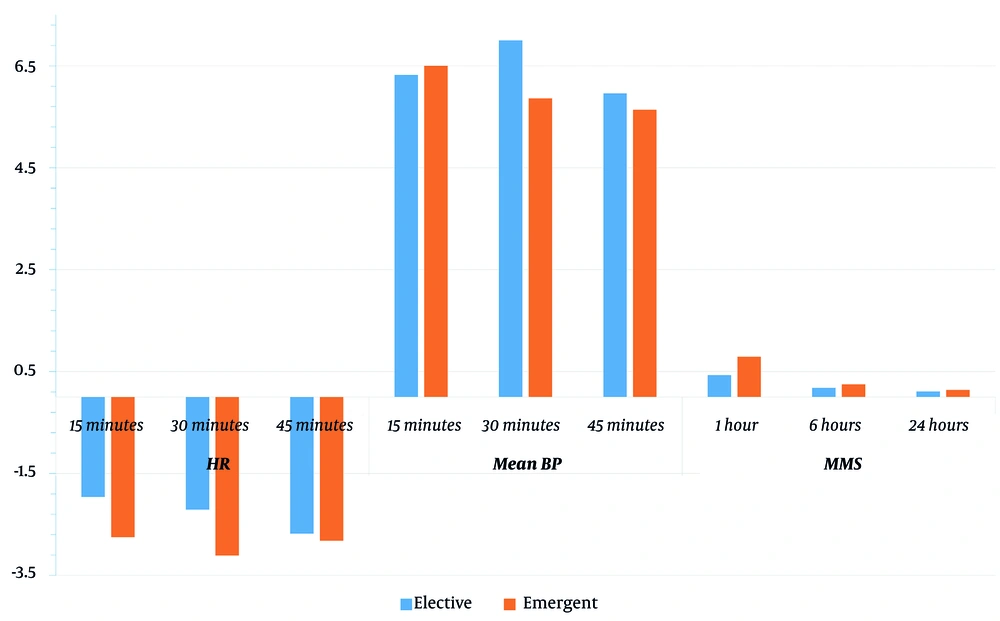
Regarding the intergroup comparison, there was a significant decline in the MBP (at 30 minutes) in the elective group compared to the emergent group (Table 3 and Figure 3).
| Parameter | Elective (n = 29) | Emergent (n = 29) | P-Value b |
|---|---|---|---|
| HR | |||
| 15 minutes | -1.96 ± 3.26 | -2.75 ± 2.55 | 0.319 |
| 30 minutes | -2.21 ± 4.03 | -3.11 ± 2.77 | 0.338 |
| 45 minutes | -2.68 ± 3.03 | -2.82 ± 2.83 | 0.856 |
| Mean BP | |||
| 15 minutes | 6.32 ± 2.39 | 6.5 ± 2.1 | 0.768 |
| 30 minutes | 7 ± 2.04 | 5.86 ± 1.41 | 0.018 |
| 45 minutes | 5.96 ± 2.74 | 5.64 ± 1.95 | 0.615 |
| MMS | |||
| 1 hour | 0.43 ± 1.29 | 0.79 ± 1.37 | 0.32 |
| 6 hours | 0.18 ± 0.94 | 0.25 ± 0.84 | 0.767 |
| 24 hours | 0.11 ± 0.57 | 0.14 ± 0.76 | 0.842 |
Differences Between Mean Values of the Study Time Intervals and the Preoperative Values a
Finally, no statistically significant difference was detected between the duration of surgery (51.43 and 50.18 minutes, respectively) or blood loss (800 and 817.86 mL, respectively) between the elective and the emergent groups (Table 1).
5. Discussion
In our comparative study, we found a significantly lower MMSE one hour after operation as compared to preoperative MMSE in the emergent group, and the MMS tended to return to normal values faster in the elective than in the emergent group. Moreover, we found a significantly lower MBP and higher HR (at 15, 30, and 45 minutes) in both groups compared to preoperative values. Furthermore, for the intergroup comparison, there was a significant decline in the MBP (at 30 minutes) in the elective group compared to the emergent group.
Indeed, stress induces more steroid secretions, whereas steroids are associated with the formation and consolidation of memory and learning (22). Additionally, steroids have a pivotal role in regulating memory in hippocampus area following acute stress (23), whereas very low or high corticosterone concentrations may weaken the learning function (24). Unlike vaginal delivery (VD), a cesarean delivery (CD) affects the duration and severity of stress at labor with less production of stress hormones. Emergency CS has incomplete labor, and the release of the stress hormones is not complete (25). The steroid concentration in umbilical cord is less in babies born by CS compared to those born by VD; it is also lower in babies born by elective CS compared to emergency CS (26, 27). Psychological stress is more likely to occur after emergent CS than an elective one (15). Subsequently, the psychological stress is related to a sense of loss, altered identity, postpartum depression, poor connection with the newborn, and emotions of aggressiveness and rage (28). This could be explained by the fact that pregnant women having elective CD are more stable than those having emergent CD (16).
Several articles investigated the effect of CS on the newborns. One report found that infants delivered by CS before labor are less excitable than babies born by VD (29). Additionally, CD significantly affects the motor development between age of 2 and 30 months (25) in addition to visual and memory impairment in babies born by CS (30). In contrast, Al Khalaf et al. found that elective CS was linked to delayed development of motor function and social skills, whereas emergent CS was associated with delay in gross motor function (31). Furthermore, Sun et al. demonstrated that elective CS affected gross motor development in 6-month-age children (32). Chan et al. declared that the impact of GA is transient since the anesthetics are cleared from the body rapidly (33). In contrast, findings from animal research strongly suggest that standard doses of routine anesthetics may produce long-term learning and memory impairments, which could persist for weeks or even months after anesthesia (6, 34). Researchers documented that CD had a sustained long-term effect on the children’s cognitive function (25), as well as memory and spatial learning due to effect on hippocampus (35).
GA influences caspase-3 activation (36), tau hyperphosphorylation (37), and beta-amyloid deposition, increasing the activity of gamma-aminobutyric acid type A receptors in the brain (2), and alters central cholinergic transmission through muscarinic and nicotinic receptors (38) leading to cognitive disturbances. Recent research also suggests that the function of gamma-aminobutyric acid type A receptor cannot return to baseline; even brief exposure to GA after the administrated anesthetic is ended (39). Each of these processes is directly associated with the development of Alzheimer’s disease (40).
The methylation of FKBP5 and NR3C1 is associated with maternal distress (41, 42). The methylation of FKBP5 is strongly linked to the cognitive function in patients who suffer anxiety disorders (43). Epigenetic alterations in FKBP5 induce altered stimulation of the hypothalamus-pituitary-adrenal axis when there was stress during adolescence (44). Parade et al. proved that methylation of NR3C1 in saliva played a role in linking early adversity and internalizing behavior alteration in children at preschool age (45).
Several recommendations have been proposed to overcome the POCD. Chan et al. concluded that bispectral index (BIS)-guided GA reduces the risk of POCD by lowering the doses of anaesthetics (33). Several studies recommended regional anesthesia to prevent POCD. Also, some studies compared GA to RA and found that early POCD can be more severe in the GA patients (46). Elderly patients experienced more severe POCD after GA (18). Saczynski et al. showed that GA increased the risk of disturbed cognitive behavior on day three after GA compared to LA (12). However, Altun et al. could not evidence a difference in MMSE scores between general and spinal anaesthesia patients (16).
Furthermore, Silbert et al. mentioned that POCD after extracorporeal shock wave lithotripsy was not influenced by the anaesthetic type (47). Another study supported these findings (48). Altun et al. concluded that POCD after short surgery is not affected by the type of anaesthetic; however, RA regional prevents risk factors of POCD such as respiratory complications and pain (16).
5.1. Limitations
The difference in early POCD between women undergoing elective or emergent CS under GA has not been investigated so far. In this study, we could not find any difference except for one hour postoperatively, maybe due to the small sample size. Future studies with larger sample sizes are needed to confirm our findings.
5.2. Conclusions
We found a significantly lower MMSE one hour after the operation as compared to preoperative MMSE in the emergent group, and the MMSE tended to return to normal values faster in the elective than in the emergent group. Moreover, we found a significantly lower MBP and higher HR in both groups compared to preoperative values. Elective CD might have a positive effect on the women's health as a mode of delivery. Future studies with larger sample sizes are required to validate our results.