1. Context
Low back pain (LBP) is the leading cause of pain and debility worldwide (1) and is the most frequent reason for work-related disability (2). Global expenditures related to LBP are staggering. Having risen exponentially over the past several decades, these expenses amount to billions of dollars each year in the United States alone (3, 4). Yet, despite the considerable healthcare resources consumed, the care provided to patients with LBP has regularly been cited as ineffective and exorbitant (5). Among the myriad reasons listed for this suboptimal care (6), the current approach to evaluation and management of patients with LBP is noteworthy and is hitherto un-investigated. The present narrative review aims to delineate the current approach, highlight problems with the existing methodology, and proposes strategies for improvement.
2. Evidence Acquisition
PubMed was searched for the keyword "non-specific low back pain". The search was restricted to articles published in English language, within the last five years, on human subjects and included only the review articles of both systematic and un-systematic category. If more than one version of the retrieved article was present, only the latest version was included. Relevant landmark articles that had shaped the modern medical concept of non-specific low back pain were also identified, by manual search and cross referencing, and were included in this review. At least two authors independently reviewed the selected articles and all authors agreed with the content of this narrative review.
3. Results
3.1. Current Methodology of Managing Patients with Low Back Pain
To understand the current methodology, it is imperative to introduce the various conditions causing LBP and examine the rationale for their prevailing categorization. Briefly, LBP is caused by a range of traumatic, inflammatory, neoplastic, metabolic, and degenerative conditions, and it can originate from diverse sources. Directly, LBP can originate from the various spinal and paraspinal structures such as the intervertebral discs (IVDs), facet joints, vertebral bodies, spinal nerve roots, spinal cord, meninges, and the surrounding muscles and ligaments. Indirectly, pain emanating from numerous structures adjacent to the spine, such as the retroperitoneal and pelvic viscera and the sacroiliac and hip joints, can also refer to the low back. For expediency, this extensive list of conditions is abridged to specific and non-specific groups (7). The specific disorders considered life-threatening and with the potential to cause serious neurologic injury have customarily included traumatic injuries, neoplasms, infections, and chronic inflammatory and metabolic conditions. In contrast, the non-specific disorders, considered benign and self-limiting, assuming their favorable natural history, encompass a wide range of vaguely defined degenerative and idiopathic conditions (7).
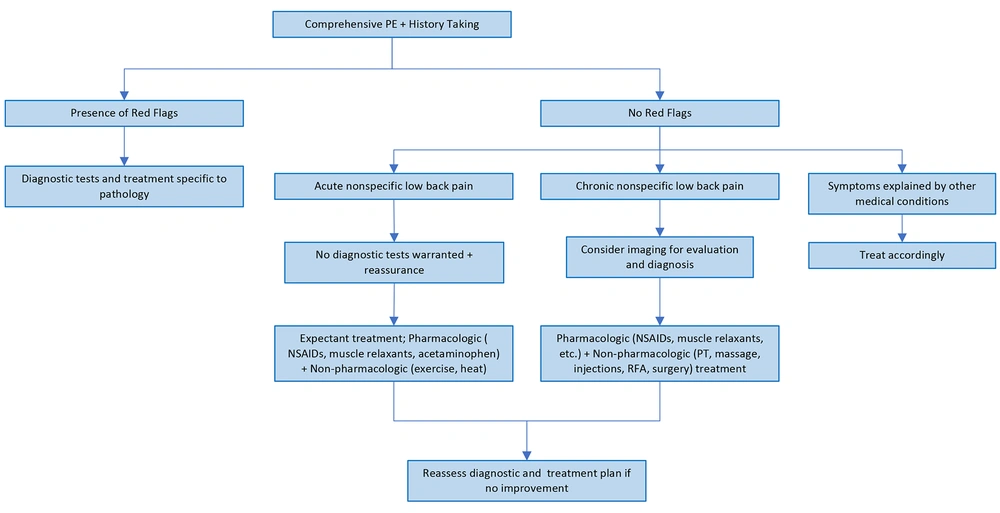
Contemporary algorithms for managing LBP first exclude the specific disorders by identifying certain signs and symptoms in patient’s history and physical examination (7). In the presence of these "red flags" (Table 1), a specific disorder is suspected, and, due to the presumed sinister prognosis, the patients are offered diagnostic work-up and explicit treatments (8). However, in their absence the LBP is presumed to be from the non-specific disorders and anticipating spontaneous recovery, the patients are managed expectantly. That is, given the diagnosis of non-specific LBP, these patients are extended no further diagnostic work-up or definitive treatments and instead are provided with generic and symptomatic therapies to control their pain and disability (7). Although in the absence of expected recovery, generally over a period of six to eight weeks, patients diagnosed with non-specific LBP may be offered further work-up and treatments (7), this recommendation is followed inconsistently, and many patients are lost to follow-up.
| Red Flag | Implications |
|---|---|
| Red Flags in the History | |
| Age younger than 20 years | Congenital and developmental disorders, spondylolisthesis |
| Age older than 50 years | Malignancy, pathologic fractures, infections, AAA |
| Short-term symptoms of < 3 months | More serious etiology |
| Trauma | Fractures |
| Fever, chills, malaise, night sweats, weight loss | Malignancy, osteomyelitis, abscess, fracture |
| History of cancer, HIV, chronic steroid use, IV drug abuse, immunosuppression | Malignancy, osteomyelitis, abscess, fracture |
| Unrelenting pain | Malignancy, osteomyelitis, abscess, fracture |
| Incontinence, saddle anesthesia, bilateral neurologic symptoms | Cauda equina compression |
| Red Flags in the Physical Examination | |
| Fever | Malignancy, osteomyelitis, abscess |
| Motor weakness, diminished reflexes, saddle anesthesia, weak anal sphincter | Cauda equina compression |
| Spinous process tenderness | Fracture |
Abbreviations: AAA, abdominal aortic aneurysm; HIV, human immunodeficiency virus; IV, intravenous.
a Source: Bigos et al. (9).
3.2. Limitations of the Current Methodology
Even though non-specific conditions imply unknown etiology, disorders procuring non-specific LBP generally have discernable etiology, pathology, and natural history. These disorders include degenerative disc disease, disc disruption, spinal stenosis, facet syndrome, herniated disc, spondylolisthesis, spinal instability, sacroiliac joint (SIJ) related disorders, and the various painful soft tissue conditions such as sprains, strains, myofascial pain, and fibromyalgia (10, 11). Furthermore, though spontaneous recovery is assumed, the natural history of these disorders is dissimilar. For instance, sprains and strains may resolve spontaneously, while disrupted discs can progress and cause widespread damage to the various spinal and para-spinal structure. These "non-specific disorders" also possess distinct risk factors and hence can be prevented, and their progression impeded, by express risk mitigation strategies. Without early recognition, employing treatments with disease modifying characteristics (12), and lacking express preventative care, a number of these "non-specific LBP disorders" will progress, causing widespread and irreversible structural damage, and will engender long-term pain and disability.
The providers caring for patients with LBP are diverse, and the list includes primary care physicians, surgeons, physiatrists, anesthesiologists, chiropractors, and osteopathic and pain management specialists. These practitioners, with different training and backgrounds, employ dissimilar treatments for analogous LBP conditions (6). This entrenched treatment variability is augmented when patients, dissatisfied with their care, without satisfactory explanation of symptoms and specific treatment plan, seek multiple opinions (5). The selection of some patients for specific treatments and others for symptomatic management, based solely on subjective clinical criteria, can also be approximate (8). Interestingly, many patients with “specific disorders”, including several traumatic, neoplastic, inflammatory, and metabolic conditions, are ultimately merely monitored, and treated symptomatically (13). Therefore, the choices of care following the current methodology may be arbitrary and these preferences can be readily swayed, for example, by the extensive publicity of the regularly introduced “novel” interventions that are generally exorbitant, invasive, and not veritably tested (5).
Overall, the current practice of cataloguing patients with LBP to specific and non-specific groups may lack significant diagnostic, prognostic, and therapeutic value. Moreover, in addition to the heightened patient dissatisfaction and the unhindered progression of certain conditions, this approach can promote inconsistent, ineffective, invasive, and repetitive care with higher costs and morbidity. Remarkably, with the specific disorders comprising less than 10%, most patients with LBP are subject to this predicament. Consequently, an alternative strategy, based on firm scientific understanding of these disorders, is warranted to standardize patient management, improve outcomes, and reduce the overall costs and prevalence of LBP.
3.3. Review of Conditions Causing Low Back Pain
To propose substantive recommendations for management of LBP, the extensive array of conditions causing it must be abridged and defined clearly. Specifically, the myriad non-specific disorders require an improved taxonomic system. Accordingly, the seemingly disparate non-specific LBP disorders can be confined, based on their shared characteristics, to just four distinct groups: (1) LBP syndromes linked to dysfunction of the IVDs; (2) soft tissue LBP syndromes; (3) pain originating from the sacroiliac joint complexes, and (4) psychosocial phenomena confounding the LBP.
3.3.1. Low Back Pain Syndromes Linked to Dysfunction of the Intervertebral Discs
Several, overtly unrelated LBP syndromes can be linked directly to dysfunction of the IVDs (14). To highlight this relationship as well as to elucidate these LBP syndromes further, the unique characteristics of lumbar IVDs, and the effects of their dysfunction on the various spinal and paraspinal structures must be examined.
3.3.1.1. Biology of Lumbar Intervertebral Discs
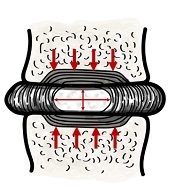
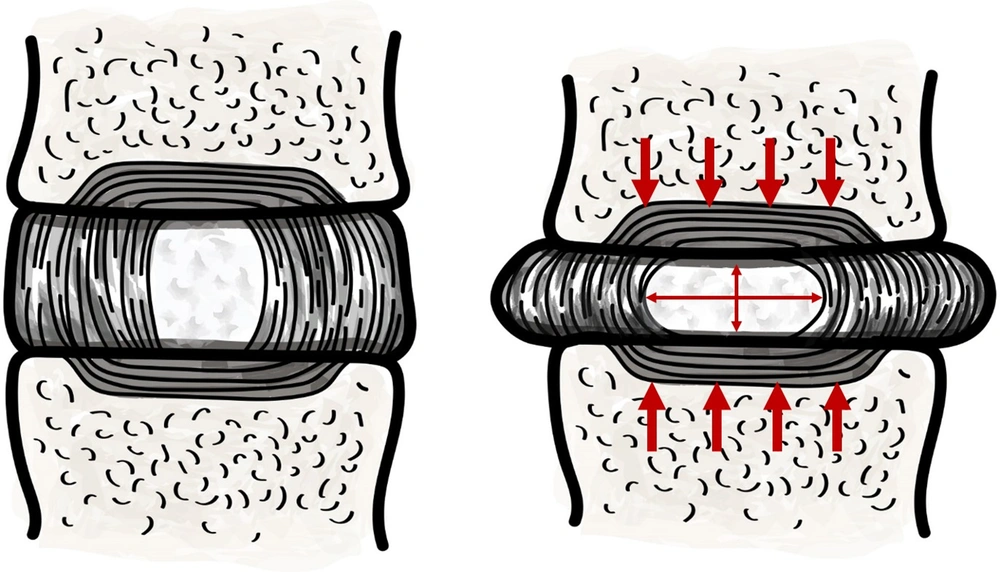
Lumbar IVD is a doughnut shaped structure interposed between the adjoining vertebral bodies. It is comprised of an outer rim, the annular fibroses (AF), and the central nucleus pulposus (NP). Both the AF and the NP are sparsely populated by cells, interspersed within ample intercellular matrix. Biophysical properties of the disc, that is, its elasticity and compressibility, depends on its matrix composition. The AF is firm in texture due to its dense matrix of interlacing collagen fibers, while the NP is jelly-like from its high matrix water and proteoglycan content (15). IVDs are highly plastic and collectively give the spine its omnidirectional range of motion. Yet, the NP conserves its height and maintains the critical gap between the adjoining vertebrae. Explicitly, the incompressible fluid (water) content of the NP, retained by the osmotic pressure generated by its proteoglycan macromolecules, dispels the axial loads as lateral stretch of the tensile AF collagen fibers (Figure 1) (16). A well hydrated NP, therefore, is integral to dissipating the immense axial loads from the contiguous structure, including the AF, facet joints, and the adjoining muscles and ligaments (17-19). The matrix composition of the disc, and hence its biophysical properties, are maintained by the anabolic activities of the disc cells and the catalytic actions of Matrix Metalloproteases (20, 21). Peculiarly, metabolic demands within the disc are met almost exclusively by intricate diffusion mechanisms, supported by complex pressure dynamics, as healthy IVDs are fundamentally avascular (16, 22).
3.3.1.2. Disc Dysfunction and Its Effects on Adjacent Spinal and Paraspinal Structures
Although structurally robust (23), IVDs are prone to dysfunction by a host of intrinsic and extrinsic influences due to their complex biology and the substantial forces endured. Amongst the extrinsic factors, most significant is the excessive physical stress on the disc, particularly from the recurring activities related to work and lifestyle, and from obesity, postural aberrations, spinal deformities, and prior spine surgery (24). Amid the intrinsic factors, polymorphism at multiple chromosomal sites has been linked to phenotypes, with variant disc constituents, that can predispose the disc to early dysfunction (24). These genetic and environmental influences can degrade the intrinsic disc environment by, for instance, reducing the anabolic activities from senescence, apoptosis, and necrosis of the disc cells, as well as by altering its enzymatic functions. The affected discs, with diminished matrix proteoglycans and collagen, are rendered progressively desiccated, fibrotic, pro-inflammatory, and non-compliant and can adversely impact the spinal dynamics. Specifically, the desiccated NP, with reduced height, exposes the AF and the adjoining muscles, ligaments, and facet joints to direct stress and injury (19, 25). The injury sustained by these structures induces local cytokine release and inflammatory cells invasion, which is followed by granulation tissue, fibrotic, vascular, and neural infiltration (26). Therefore, thru enduring risk factors, and the ensuing disc dysfunction, the affected discs, facet joints, vertebral bodies, and the local spinal and paraspinal muscles and ligaments can undergo varied degrees of inflammation, fibrosis, neo-innervation, neo-vascularization, and hypertrophy. These pathological changes can cause LBP both directly and indirectly by compromising the in-housed neural structures.
3.3.1.3. Low Back Pain Syndromes Linked to Lumbar Disc Dysfunction
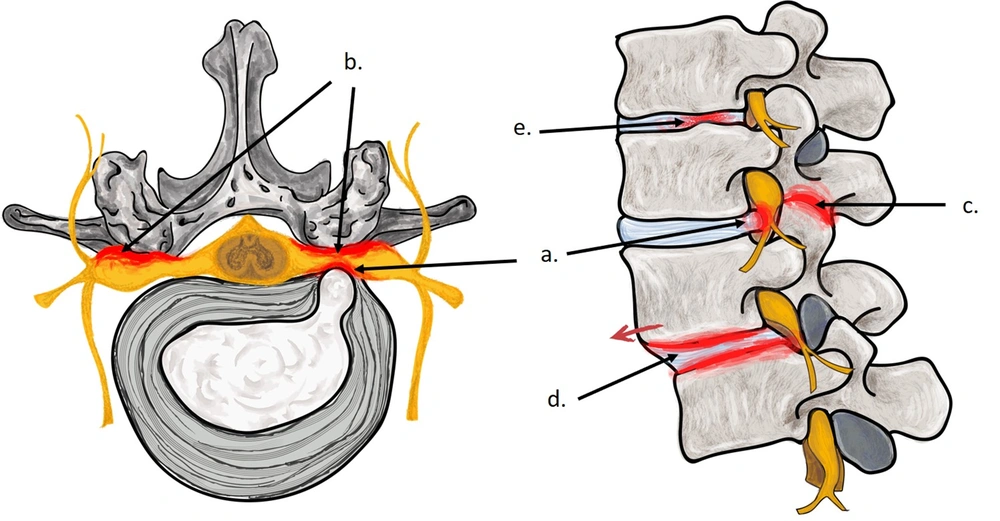
The local injury and repair responses from dysfunction of the affected discs, in time, can progress to many well-recognized LBP syndromes (Figure 2). The hallmark feature of internal disc disruption are the AF tears resulting from exposure to direct stress and injury. These AF tears, highly vascularized, richly innervated, and replete with nociceptors, provide ready substrate for pain especially when exposed to the pro-inflammatory disc contents—a chemically sensitive disc on discography (27, 28). Desiccated discs have lower signal intensity and reduced height, and they show generalized bulging on spinal imaging. These findings often labelled as degenerative disc disease, are frequently encountered in patients with LBP as well as in asymptomatic individuals. Therefore, they are often considered both a source of chronic pain and an age-related physiologic variant (23, 25). In herniated disc, the extrusion of disc contents typically occurs via AF tears in discs subject to dysfunction, and the pain is caused from the disc pathology as well as from the interface of pro-inflammatory disc contents with contiguous neural structures (17). The facet joints constraint the tortional forces while the axial stress is borne by the IVDs. Therefore, direct stress of the facet joint, from the reduced disc height, can render them arthritic and hypertrophied (29, 30). In lumbar facet syndrome, LBP results from the arthritic facet joints as well as from neural compromise caused by their hypertrophy and resultant narrowing of the contiguous spinal canal and neuroforaminae (29, 30). Spinal stenosis most frequently is of degenerative category, and the narrowing of the spinal canal and neuroforaminae, from bulging of the AF and hypertrophy of the facet joints and ligamentum flavum, typically occurs at the disc levels. Disc dysfunction can also weaken the disc structure, permitting creep, that can instigate both spinal instability and spondylolisthesis, both again generally of degenerative variety (29). Therefore, even though several LBP syndromes are regularly considered independently, they frequently emanate directly from dysfunction of the lumbar IVDs.
3.3.1.4. Characteristics of Pain Generators Linked to Lumbar Disc Dysfunction
Due to their common origin, LBP syndromes consequent to disc dysfunction are generally present concomitantly. Yet, their presentation is typically asymmetrical with various permutations. For example, though concurrent, a herniated disc may be the main cause of pain in some patients while an internally disrupted disc may be the predominantly source in others. Similarly, degenerated discs, facet syndrome, spinal stenosis, spinal instability, and spondylolisthesis frequently coexist and variably cause patient’s LBP. Chronologically, the incongruities in injury and repair cycles can result in LBP that is typically episodic nature, with periods of remission and relapses. However, progressive disc dysfunction generally results in worsening LBP over time and truncating periods of remission (23). The injury and repair cycles also explain the poor correlation of symptoms with changes seen on spinal imaging (i.e., the pain may abate due to the local reparative processes even though the morphological changes incurred persist (23)). Overall, disc dysfunction can engender multiple, concurrent, inconstant, and recurring pain generators that correlate poorly to the morphological changes.
Recognition of the pertinent pain generators, due to disc dysfunction, with these vacillating characteristics, can be challenging. Amongst the available diagnostic tests, MR imaging of the lumbar spine is most frequently utilized. However, as alluded to earlier, its findings can be similar in both symptomatic as well as in asymptomatic individuals (31). Other commonly employed diagnostic tests include local anesthetic blocks (e.g., facet joint and nerve root injections) and provocative testing (e.g., discography). However, these diagnostic modalities can also be non-specific due to their subjective nature (32). Accordingly, delineation of relatable pain generators linked to disc dysfunction requires clear understanding of the causative conditions, meticulous clinical evaluation of the patients, judicious use of the diagnostic tests, and correlation of the clinical findings with the diagnostic results.
3.3.1.5. Management of Syndromes Linked to Disc Dysfunction
Treatments of LBP syndromes linked to disc dysfunction, in addition to the symptomatic therapies, generally include a range of minimally invasive and surgical interventions (33-35). These treatments typically seek diminished inflammatory response, removal of herniated disc material and/or disrupted discs, neural decompression, spinal stabilization, and correction of the spinal deformities. However, these interventions cannot impede the progressive damage from disc dysfunction and the relief in LBP, therefore, is often incomplete and short-lived. Similarly, attempts to restore the disc matrix composition by employing various cell and growth factor-based therapies remain exploratory (36). Given the immense physical stresses endured by the disc and its anaerobic and pro-inflammatory intrinsic environment, the prospects of success for these regenerative therapies may also be limited. Overall, the available treatments are unable to halt the evolving disc dysfunction and, therefore, have limited capacity to resolve, or even arrest, the damage incurred to the disc and the contiguous structures (33-35). In this void, early recognition and containment of disc dysfunction by adopting specific risk mitigation strategies is paramount. Accordingly, protocols must be modeled and implemented, in both high-risk individuals and in patients with LBP, to limit the progressive structural damage from disc dysfunction (24).
Even though altered spinal dynamics from disc dysfunction can provoke number of soft tissue and SIJ related pain conditions, these LBP syndromes, and the accompanying psychosocial phenomenon, can also develop autonomously and are therefore discussed independently (37).
3.3.2. Soft Tissue Low Back Pain Syndromes
Soft tissues of the low back include local muscles, their fascial layers, tendons, aponeurosis, and their insertions, as well as the local ligaments. Injury to these soft tissues is a common source of both acute and chronic LBP. Although direct insults such as spine surgery can cause chronic LBP, pain of soft tissue origin most frequently results from a wide range of indirect stresses (38). Accentuated by the body weight and postural aberration, these indirect insults include: (1) altered spinal dynamics (e.g., from the underlying IVD or SIJ pathology); (2) neurological conditions (e.g., spinal cord injury and spastic disorders); (3) spinal deformities (e.g., kyphosis and scoliosis); (4) prior spine surgery –spinal fusion, in particular, and (5) gait and lower limb abnormalities (e.g., leg length discrepancies and hip, knee, and ankle joint disorders). LBP of soft tissue origin is also predisposed by genetic susceptibility, malnutrition, poor conditioning, psychological stresses, and exposure to noxious substances (e.g., drugs, alcohol, and tobacco (11, 39)).
Muscle injuries, analogous to other highly vascular tissues, initiate local inflammatory reaction that is followed by competitive muscle restoration characterized by recruitment of native and satellite stem cells and local fibrosis. Therefore, whereas acute muscle injuries can heal without consequence, chronic muscle injuries may accompany local fibrosis, loss of muscle mass, and partial recovery of muscle strength (26). Contrary to the low back muscles, local tendons, ligaments, aponeurosis, as well as their osseous insertions are relatively avascular and can heal sluggishly and erratically under chronic stress. The altered regional dynamics resulting from these chronic soft tissue injuries can accompany inflammatory reactions in the associated fascial layers and altered composition of the fascial lubricants, causing local myofascial pain (40).
The soft tissue syndromes affecting the low back can be acute, chronic, localized, or widespread, and may or may not accompany the various psychosomatic phenomenon. Often imperceptibly distinct, these soft tissue LBP syndromes include muscular and ligamental sprains and strains, myofascial pain syndrome, and fibromyalgia (11, 38). Muscular sprains and ligamental strains generally cause acute and short-term local pain and tenderness that typically resolves within days to weeks (26). Similarly, acute and localized form of myofascial pain syndrome can resolve spontaneously, whereas its chronic and generalized variety may be intractable and recalcitrant to treatment (41, 42). In fibromyalgia, widespread pain and tenderness is associated with central and psychogenic phenomenon, including fatigue, sleep, memory, and mood disturbances (41). The diagnosis of these soft tissue LBP syndromes is based primarily on self-reported symptoms, while their treatment is fundamentally symptomatic in nature, including medications of various categories (42), physical and psychological therapies, and local modalities such as regional stimulation, acupuncture, massage, and trigger point injections (41, 42).
3.3.3. Pain Originating from Sacroiliac Joint Complexes
The paired SIJs transfer the full axial load of the body from the spine to the lower extremities. SIJs possess a thick fibrous capsule, are only partially synovial, and have a complex web of muscles, ligaments, tendons, and their insertions into the respective bony structures. Many of these joint constituents are poorly vascularized and, therefore, heal sluggishly, yet, most are richly innervated by nociceptive fibers and can be an eminent source of pain. With these characteristics, substantial strains endured, and the synovial make-up, SIJs are predisposed to chronic pain and can be afflicted by a range of pathological conditions.
Traumatic injuries to the SIJs are prevalent and include both the isolated direct impacts (e.g., falls and motor vehicle accidents) and the more frequent, indirect, and repetitive stresses related to altered spinal and gait dynamics discussed earlier. Accordingly, pain from SIJs generally accompany other low back, lower extremity, and neurological disorders (43). Injuries to the SIJs include capsular, synovial, and ligamental disruptions, enthesopathy, chondromalacia, muscular injuries, and macro and micro fractures. Overtly, only minority of these injuries are discernable by the imaging studies (44). SIJs are also implicated in a range of inflammatory spondylarthropathies and are frequent site of primary and secondary neoplasms. Isolated SIJ pain is also common during pregnancy, predisposed by the hormone induced ligamental laxity, weight gain, lordotic posture, and the mechanical trauma of parturition. Pregnancy induced SIJ pain frequently persists in the postnatal period.
Clinical evaluation of patients with SIJ pain can be non-specific as other regularly coexistent sources of pain engender similar clinical findings. Similarly, as mentioned earlier, results of SIJ imaging, including X-Rays, CT, and MR, are frequently unremarkable and correlate poorly (45). Although pain relief from targeted local anesthetic SIJ injection has emerged as an exclusive confirmatory test, its diagnostic accuracy has been challenged due to the subjective nature and high false positive rate. Treatments for SIJ pain, including medications, physical therapy modalities, minimally invasive techniques, and surgical fusion, are also mainly symptomatic and have limited long-term efficacy (46-49).
3.3.4. Psychosocial Phenomena Confounding Low Back Pain
Chronic LBP syndromes can perpetuate a cycle of local pain and tenderness that can heighten spinal and supra-spinal plasticity and can promote antidromic neurogenic inflammatory reactions (26, 50). These local and central phenomena can trigger regional and generalized hyperalgesia, allodynia, and other neuropathic phenomena, augmenting patients' chronic LBP (11). The altered central pain processing in the long-term can also propagate several psychosomatic phenomena, including fatigue, insomnia, and other psychological and behavioral changes (11). Among the psychological disorders encountered in patients with chronic LBP most prevalent are depression, anxiety, post-traumatic stress, substance abuse, and somatization (51-53). The corresponding social issues include inability to work, disability, drug dependency, medico-legal disputes, and multiple other secondary gains (53). These psychosocial phenomena, common in LBP patients, can precede and may have a causative role, but more frequently they ensue LBP and, therefore, play a substantial role in perpetuation. Accordingly, the psychosocial phenomena accompanying LBP must be recognized in a timely manner and remedied by a multidisciplinary approach to optimize patient outcomes.
4. Discussion
LBP remains a leading global public health problem despite utilization of enormous healthcare resources. Among the myriad reasons for this discouraging outlook, the current methodology of evaluation and management of patients with LBP may be a major contributor. In summary, patients with LBP are primarily treated empirically, anticipating spontaneous recovery, without specific diagnosis, treatment, or explicit preventative care. Yet, many LBP disorders are progressive and can cause long-term pain and debility by irreversibly damaging the various spinal and para-spinal structures. Therefore, early recognition of these LBP disorders, currently labelled as “non-specific”, is critical to prevent the chronic pain, disability, neural compromise, and the attendant psychosocial impacts.
Explicitly, except for the acute soft tissue muscular and ligamental strains, sprains, and acute myofascial pain, most LBP disorders, both specific as well as non-specific, require precise diagnosis and treatment. As elaborated in the main article, with the treatments for most LBP disorders lacking disease modifying characteristics, early detection, and prompt implementation of preventative strategies, impeding the progression of these LBP syndromes may currently be the only viable option to improve patient outcomes.
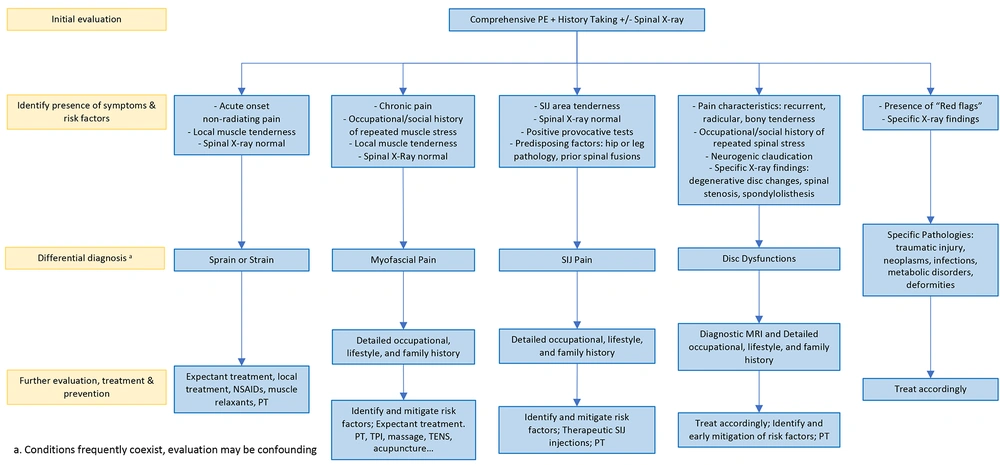
To achieve this goal, we propose that patients with LBP are assessed and managed comprehensively, analogous to patients with most other clinical conditions. Specifically, these patients should be evaluated not only for the absence of the traumatic, infectious, neoplastic, and inflammatory conditions but for their presence and also for the existence of disorders related to disc dysfunction (i.e., degenerative disc disease, internal disc disruption, herniated disc, facet arthritis, spinal stenosis, and spondylolisthesis), the various chronic soft tissue and SIJ related LBP syndromes, and for the associated psychosocial phenomena. Accordingly, contrary to merely eliciting the "red flags", the clinical assessment of patients with LBP should entail thorough clinical evaluation and precisely employed diagnostic tests.
Following this paradigm, when the clinical evaluation is suggestive of acute soft tissue sprains, strains, and acute myofascial pain, further diagnostic work-up may be deferred. However, strategically employed diagnostic tests are necessary in all other instances. For example, when indicated, a spinal roentgenogram would exclude most traumatic, infectious, inflammatory, neoplastic, metabolic, and degenerative conditions, especially when the osseous structures are involved (54). However, cross-sectional imaging, using MR or CT, is obligatory to clearly demarcate lesions within the spinal canal and to delineate the extent of disc dysfunction and the contiguous disc and other structural involvement (35). A focused clinical evaluation within this contextual framework can determine the plausible pain generators in most patients. However, pertinently selected diagnostic blocks and provocative tests may be useful adjunct in challenging situations (Figures 3 and 4).
The strategy of precise and early diagnosis discussed here may have higher preliminary costs, however, the eventual care expenditures would be substantially lower. For instance, early and specific diagnosis can avoid the generic, ineffective, and repetitive care, and preclude disease progression through early prevention, and may avert morbidly invasive and exorbitant interventions.
Ultimately, the most pragmatic way to reduce the staggering morbidity and costs of LBP depends on comprehensive understanding of the causative conditions, systematic approach to their early recognition, early deployment of disease modifying treatments when available, and prompt mitigation of risk factors identified by tailored preventative care approach.
5. Conclusions
It was concluded that LBP remained a significant and unresolved public health problem. The ongoing approach of managing patients with LBP was found to likely contribute to this grim outlook. The alternative approach, presented in this article, may improved patient outcomes.