1. Background
Although most cholecystectomies are performed using a laparoscopic technique, post-laparoscopic cholecystectomy (LC) pain control is still an issue (1-3). Unlike other laparoscopic procedures, pain after LC can be moderate or even severe for patients and may require opioid treatment (4). Pain after LC alone is the second reason for 30-day hospital readmission after surgical complications (5). Early pain is a prevalent complaint after LC and is often responsible for an overnight stay on the day of admission in 26 - 41% of patients (6-8). Post-LC visceral pain is an independent risk factor for chronic, unexplained pain (9).
Post-LC pain is multifactorial; thus, multimodal analgesia has been suggested for its treatment (1, 10). Visceral pain has been the primary source of postoperative pain in LC, especially in the first 24 hours (4, 11). The primary source of this pain is believed to be the visceral surgical manipulations during surgery, including clamping the cystic duct and cystic artery and resectioning the gall bladder. Coughing can trigger this pain and increases its intensity (12-15). Somatic or parietal pain in LC is less intense than visceral pain due to the small abdominal incisions (often 1 to 4 cm) of the trocar site and the limited damage to the abdominal wall (16). With more attention to regional anesthesia as part of multimodal analgesia (17), different techniques have been used for post-LC pain control, such as paravertebral block (18-20), rectus sheath block (21), transversus abdominis plane block (22-24), intercostal nerve block (25), subcostal transversus abdominal plane block (STAP) (26), thoracic epidural (27, 28), and erector spinae plane block (ESPB). Bilateral ESPB has received significant attention as a regional technique for post-LC analgesia. Bilateral ESPB has been compared to STAP with similar or superior analgesia after LC (29-31). Case reports (4, 32, 33) and randomized control trials (34-38) have shown the efficacy of bilateral ESPB for pain control after LC, though the effects of unilateral ESPB have not yet been studied.
The recent finding of abdominal visceral pain control of ESPB (38-40) and bilateral sensory block effects of single unilateral injection by Schwartzmann et al. (41) and Tulgar et al. (42) has raised the question of whether unilateral ESPB can be used for postoperative pain control after LC.
2. Objectives
This study aimed to compare the right-sided unilateral ultrasound-guided ESPB with patients using intravenous narcotic analgesia on the post-LC analgesia consumption and pain in a single-center randomized controlled trial. It was hypothesized that adding right-sided unilateral ESPB after LC would reduce the intensity of pain and opioid consumption and increases the patients’ satisfaction with pain control.
3. Methods
The Institutional Ethics Committee of Iran University of Medical Sciences approved the study protocol (code: IR.IUMS.FMD.REC.1397.287). Written informed consent was obtained from all subjects. The study was registered on the Iranian Registry of Clinical Trials website (code: IRCT20120814010599N25).
3.1. Eligibility Criteria
This prospective, single-center, single-blinded parallel arm randomized controlled clinical trial was performed on patients who were candidates for elective LC and referred to a quaternary university-based hospital in Tehran, Iran, between 5 February and 10 October 2020. Patients were included if they were between 20 and 65 years of age with an American Society of Anesthesiologists (ASA) physical status of I or II who were planning to undergo an LC. Patients were excluded if they were undergoing an emergency cholecystectomy, had a body mass index greater than 35 kg/m², had a history of opioid dependence/tolerance, had an allergy to ropivacaine, and had liver or renal disease or coagulopathy. Patients were further excluded intraoperatively if the surgery was converted to an open cholecystectomy or if another problem occurred intraoperatively (eg, uncontrolled intraoperative bleeding).
3.2. Randomization and Blinding
Eligible patients were randomly assigned to 2 groups (the block group [BG] and the control group [CG]). The randomization was generated by an independent statistician at the clinical research development center using a simple random sequence with permuted blocks (http://www.randomization.com) of size 4 in a 1:1 allocation. Each patient’s assignment was sealed in an opaque, sequentially numbered envelope. The envelope was opened
in the post-anesthesia care unit (PACU) by either of the 2 regional anesthesia study staff members (PH or SHRF) to administer the block (or control). Separately, all individuals assessing outcomes were blinded to group assignment.
3.3. Standards of Care
Patients in both groups were anesthetized per the research protocol in accordance with the institutional standards of care, including intravenous antibiotic prophylaxis according to the hospital’s protocol, in which 2 µg/kg of fentanyl and 0.12 mg/kg of midazolam were used as premedication. All patients were monitored by ASA standard monitoring and bispectral index (BIS). Anesthesia was induced using 2 mg/kg of propofol and 0.2 mg/kg of cisatracurium. Total intravenous anesthesia was used, including 100 - 150 µg/kg/min of propofol, 2 mg of cisatracurium (as needed), and 50 mcg of fentanyl every 30 minutes (as needed). End-tidal carbon dioxide was kept between 30 to 35 mm Hg, and BIS was maintained between 40 and 60. Four surgical trocar entry points (measuring between 1 and 5 cm) were used: one at the umbilicus and three in the right upper abdominal quadrant. Also, 4 mg of ondansetron and 1 g of acetaminophen were given in the last 20 minutes intravenously. After evacuating the pneumoperitoneum, cisatracurium was reversed by intravenous neostigmine (0.04 - 0.07 mg/kg) and atropine (7 µg/kg), and the patient was extubated. In both groups, standardized monitoring was applied on arrival at PACU. In addition, patient-controlled intravenous analgesia (PCIA) containing 6 µg/mL of fentanyl with a continuous infusion of 2 mL/h and bolus bottom (2 mL every 15 minutes, as needed) was started for each patient upon arrival to the PACU. At such a low dose, the half-life of fentanyl is very short; therefore, the continuous infusion looks safe, even in narcotic naïve patients. However, the patient did have an option of PCIA of another 12 mcg fentanyl every 15 minutes (lock-out interval) PRN to treat the pain. This makes the maximum dose of 50 µ per hour, which is very routine for PACU pain control, even in narcotic naïve patients.
3.4. Study Interventions
ESPB (43) was performed upon arrival to the PACU for patients in BG by 2 experienced regional anesthesiologists (PR, SHRF). For the ESPB, the patient is positioned on the left lateral with the right side up. The spinous process of the seventh thoracic vertebra (at the level of the lower border of the scapula) was marked. After sterilization, using a high linear frequency (5 - 13 MHz) ultrasound probe (Fujifilm Sonosite S-Nerve, Bothell, WA, USA), the right-sided seventh transverse process (TP) was recognized. Then a 22-gauge 90 mm disposable spinal needle was inserted and arrived at the tip of the seventh TP. A total of 20 mL of 0.2% ropivacaine was injected with increments of 5 mL with negative aspiration each time. The accurate site of the injection point was controlled by detaching the erector spinae muscles from the TP. The success of the ESPB was confirmed by obtaining ultrasound images of the craniocaudal spread and subsequent multi-dermatomal thermal changes to the cold stimulus that the patient perceived on the right-sided dorsolateral aspect of the mid-thorax.
3.5. Outcomes Assessment
The primary outcome of interest was patient-reported pain intensity using a Numerical Rating Scale (NRS) during rest and voluntary deep coughing. This score resulted in a value from 0 (no pain) to 10 (severe pain) and was assessed by a blinded outcome assessor at 7 different times, including on arrival to the PACU (time 0), as well as at 20 minutes and 2, 4, 6, 12, and 24 hours after admission to the PACU. For each patient with NRS greater than 3, 20 mg of intravenous meperidine was prescribed as rescue analgesia every 3 to 4 hours during the postoperative period for 24 hours, as needed. Meperidine was chosen as a rescue drug because meperidine produces less spasm in the sphincter of Oddi than other opioids due to its atropine-like effects; therefore, meperidine is an appropriate choice for the treatment of biliary tree and pancreatic pain.
The same blinded outcome assessor evaluated the patient’s satisfaction with their pain control at each interval and the end of 24 hours using a 5-point satisfaction score (0, weak; 1, moderate; 2, good; 3, very good; 4, excellent) as an exploratory endpoint. Other secondary outcomes included the total opioid consumption used during the first 24 hours after surgery (in mg).
3.6. Sample Size Determination
The sample size determination was based on the previous studies on the effect of the ESPB on post-LC analgesia (36). The study was powered to detect a difference of 2 points in pain with an SD of 0.93 points, assuming a 2-tailed alpha of 0.05 and power of 0.80, which required a sample size of 62 patients (31 patients in each group). Considering the possibility of a 10% drop, a total of 68 patients were considered for the study.
3.7. Statistical Analysis
Descriptive statistics were reported as mean ± SD or medians (interquartile ranges [IQR]) for numeric variables based on the data distribution. Normality was assessed using the Shapiro-Wilk test and confirmed by a visual inspection of the data.
Categorical variables were summarized using frequencies and percentages. Univariate analyses were performed using 2 independent sample t-tests for normally distributed data, Wilcoxon rank sum tests for non-parametric continuous variables, and chi-square and Fisher exact tests for testing associations of categorical variables. To evaluate pain scores over time between groups, separate generalized linear models (with Gaussian distribution and identity link function) were constructed for pain scores at rest and with coughing. In each model, the patients’ study number was included as a random intercept to account for the repeated nature of data collection. Models were constructed with a fixed effect for group assignment and time of pain score collection (20 minutes and 2, 4, 6, 12, or 24 hours); models were conditional on the baseline pain score. The results of these models are presented as mean differences and their associated 95% CI. All statistical analyses were performed using SPSS version 21 (SPSS Inc, Chicago, Ill, USA) and SAS version 9.4 (SAS Institute Inc, Cary, NC). Two-sided P-values of less than 0.05 were considered statistically significant for all analyses.
4. Results
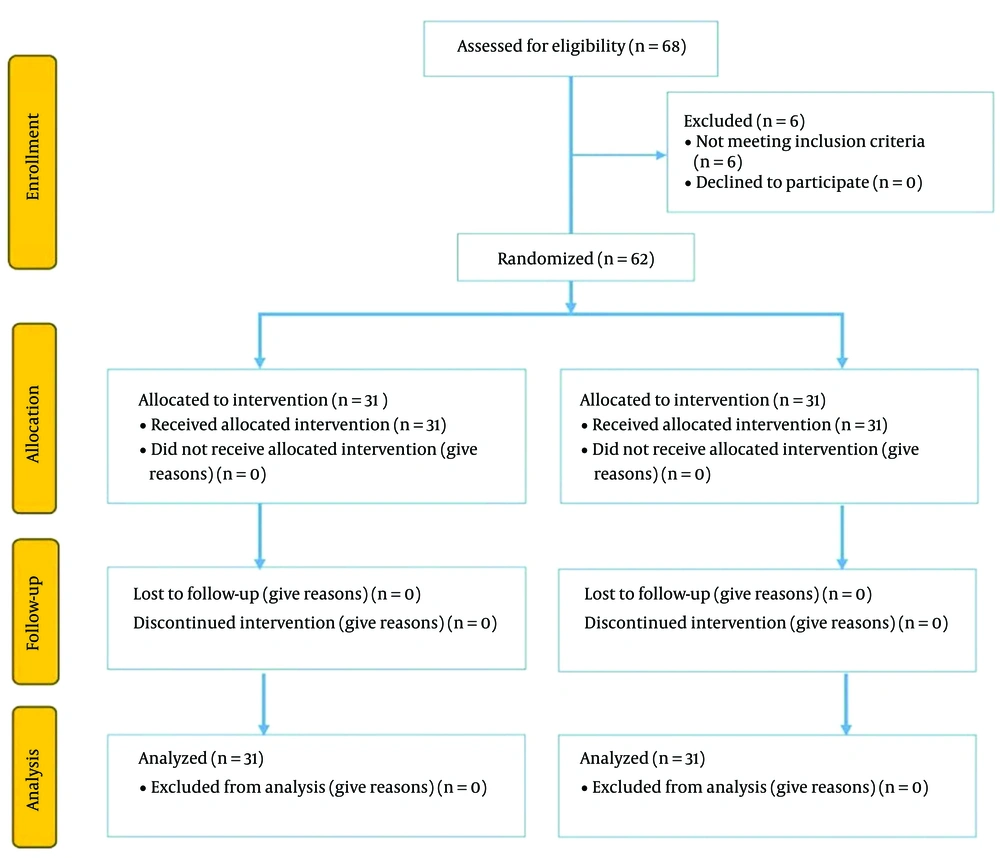
Sixty-eight patients undergoing elective LC were enrolled in the study. Six patients were excluded from the study because they converted to open surgery and vessel injury intraoperatively. Thus, a total of 62 patients were randomized postoperatively and included in the analysis of this study (Figure 1). Age, body mass index, ASA status, intraoperative fentanyl usage, and operation time were not statistically different between the 2 groups (Table 1).
| Block Group (n = 31) | Control Group (n = 31) | P-Value | |
|---|---|---|---|
| Patient characteristics | |||
| Age, y | 45.6 ± 12.4 | 40.9 ± 9.5 | 0.10 |
| Sex (female) | 25 (80.65) | 26 (83.87) | 0.74 |
| Weight, kg | 70 (67, 76) | 70 (60, 80) | 0.48 |
| Height, cm | 162 (160, 169) | 160 (158, 165) | 0.12 |
| Body mass index, kg/m2 | 26.3 (25.4, 27.8) | 25.4 (24.0, 30.5) | 0.78 |
| ASA physical status I (%) | 22 (70.97) | 22 (70.97) | > 0.99 |
| Surgical characteristics | |||
| Operation time, min | 115 (80, 140) | 120 (80, 120) | 0.67 |
Abbreviation: ASA, American Society of Anesthesiologists.
a Data are reported as mean ± SD, median (interquartile range) or n (%) depending on the variable type and distribution.
4.1. Pain Scores
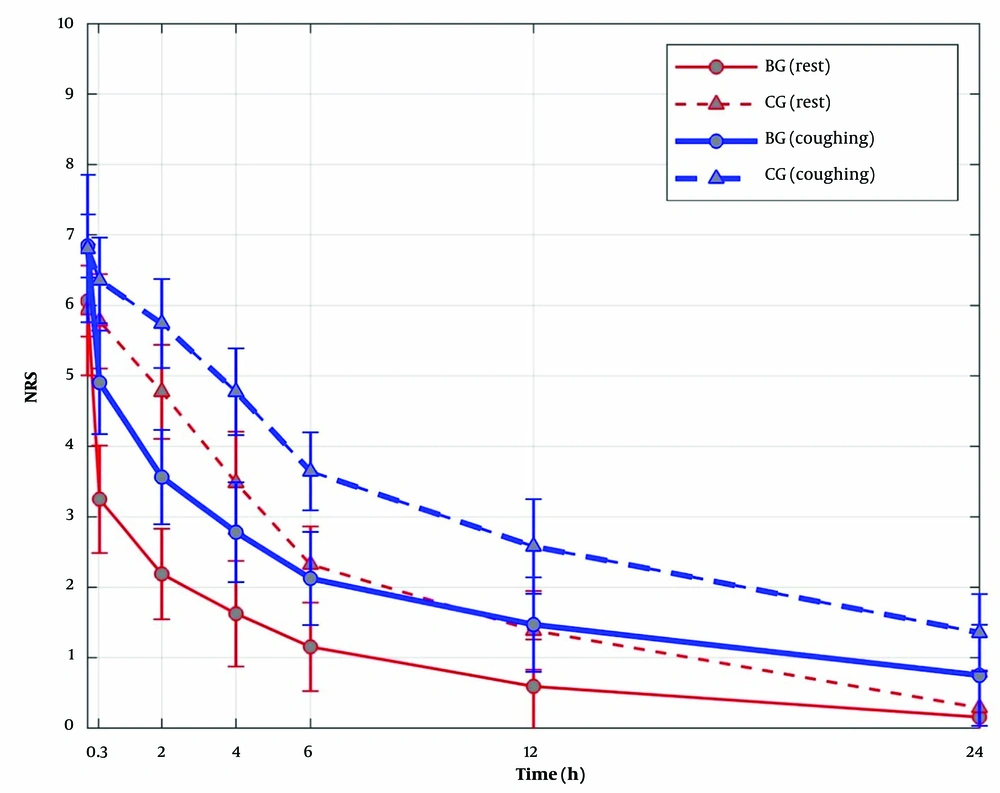
Pain scores both at rest and during a cough at the time of arrival in the PACU were not statistically different between the 2 groups. Patients in BG had lower pain scores both at rest (mean difference -1.54 points; 95% CI, -1.78 to -1.29; P < 0.0001) and during coughing (mean difference -1.51 points; 95% CI, -1.74 to -1.29; P < 0.0001) compared with CG. A statistically significant interaction was observed, such that this association varied by time (P < 0.0001 for at rest and with cough models; Figure 2). Specifically, differences were observed up to 12 hours postoperatively. That is, in the first 12 hours after arrival to the PACU, a difference in median (IQR) NRS pain scores was observed at rest (BG 1 [0, 1] vs CG 1 [1, 2]) and with coughing (BG 1 [1, 2] vs CG 2 [2, 3]) in BG vs CG, respectively (both P < 0.0001). At 24 hours, pain intensity with cough was slightly higher in CG (median 1 [IQR 1, 2] vs 1 [1, 0]; P = 0.0005) than in BG; however, no statistically significant difference was observed at rest (BG 0 [0, 0] vs CG 0 [0, 1]; P = 0.12). In BG, 71.0% of patients had NRS pain scores less than 4 at 20 minutes after surgery, and by 6 hours, 87.1% of patients in BG had pain scores below 2. In CG, no patients (0%) had NRS pain scores less than 4 at 20 minutes after surgery, and only 1 patient (3.2%) had a pain score below 2 at the 6-hour time point.
4.2. Opioid Consumption and Other Secondary Outcomes
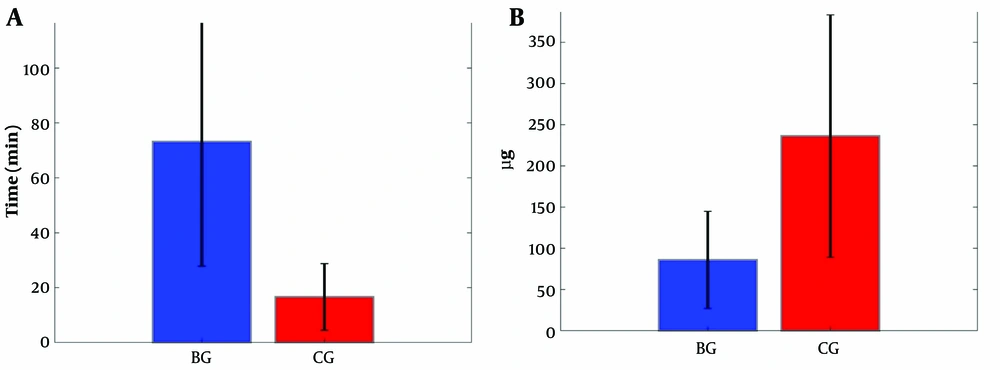
At 24 hours, the total opioid (fentanyl) consumption was significantly reduced in BG than in CG (median 60 µg [IQR 60, 90] vs 250 µg [90, 300]; P < 0.0001; Table 2). Overall, 77.4% of patients in BG consumed less than 100 µg of fentanyl, while 78.0% of patients in CG required more than 100 µg of fentanyl. Of note, no patient in BG required more than 300 µg of fentanyl. In contrast, 22.6% of patients in CG required PCIA to be recharged because they required more than 300 µg of fentanyl in the first 24 hours postoperatively. Further, the time to first analgesia request was significantly faster in CG than in BG (median 10 minutes [IQR 10, 20] vs 80 [55, 110]; P = 0.0002; Figure 3). The mean meperidine dose administered as rescue analgesia in the first 24 hours after surgery was also significantly lower in BG than in CG (median 20 [IQR 10, 20] vs 25 [20, 25]; P = 0.002).
| Block Group (n = 31) | Control Group (n = 31) | P-Value | |
|---|---|---|---|
| 24-Hour opioid consumption, mg | 60 (60, 90) | 250 (90, 300) | < 0.0001 |
| Time to first request, min | 80 (55, 110) | 10 (10, 20) | 0.0002 |
| 24-Hour meperidine dose, mg | 20 (10, 20) | 25 (20, 25) | 0.002 |
| Pain scores at rest | |||
| PACU arrival (time 0) | 6 (6, 6) | 6 (6, 6) | 0.82 |
| 20 min | 3 (3, 4) | 6 (6, 6) | < 0.0001 |
| 2 h | 2 (2, 2) | 5 (4, 5) | < 0.0001 |
| 4 h | 2 (1, 2) | 4 (3, 4) | < 0.0001 |
| 6 h | 1 (1, 1) | 2 (2, 3) | < 0.0001 |
| 12 h | 1 (0, 1) | 1 (1, 2) | < 0.0001 |
| 24 h | 0 (0, 0) | 0 (0, 1) | 0.12 |
| Pain scores during cough | |||
| PACU arrival (time 0) | 7 (7, 7) | 7 (7, 7) | 0.54 |
| 20 min | 5 (4, 5) | 6 (6, 7) | < 0.0001 |
| 2 h | 4 (3, 4) | 6 (5, 6) | < 0.0001 |
| 4 h | 3 (2, 3) | 5 (4, 5) | < 0.0001 |
| 6 h | 2 (2, 2) | 4 (3, 4) | < 0.0001 |
| 12 h | 1 (1, 2) | 2 (2, 3) | < 0.0001 |
| 24 h | 1 (0, 1) | 1 (1, 2) | 0.0005 |
| Satisfaction scores | |||
| PACU arrival (time 0) | 0 (0, 0) | 0 (0, 0) | 0.17 |
| 20 min | 2 (2, 2) | 0 (0, 0) | < 0.0001 |
| 2 h | 2 (2, 3) | 1 (1, 1) | < 0.0001 |
| 4 h | 3 (2, 3) | 1 (1, 1) | < 0.0001 |
| 6 h | 3 (3, 3) | 2 (1, 2) | < 0.0001 |
| 12 h | 3 (3, 3) | 2 (2, 3) | < 0.0001 |
| 24 h | 3 (3, 4) | 3 (3, 3) | 0.0002 |
Abbreviation: PACU, post-anesthesia care unit.
a Data are reported as mean ± SD, median (interquartile range) or n (%) depending on the variable type and distribution.
Postoperative opioid consumption. (A) Time to first analgesia (meperidine) was significantly lower in the block group than in the control group (P = 0.0002). (A) The fentanyl dose consumption in the first 24 hours postoperatively was statistically higher in the control group (P < 0.0001) than in the block group. Abbreviations: BG, block group; CG, control group.
After PACU arrival, differences in patient satisfaction were observed 24 hours postoperatively. Patients in BG reported higher overall satisfaction with their pain control, reporting median scores of “very good” compared with CG. The median satisfaction score in BG was 3 (IQR 3, 4) compared to 3 (IQR 3, 3) in CG (P = 0.0002). In BG, 83.9% of patients achieved “good” satisfaction (score of 2 or higher) at 20 minutes, whereas no one in CG achieved this metric within the first 20 minutes of PACU arrival. By 6 hours, 96.8% of patients in BG achieved “good” or better satisfaction (6.5% excellent, 80.7% very good, and 9.7% good), whereas only 67.7% of the CG achieved “good” satisfaction (3.2% very good and 64.5% good).
4.3. Safety and Adverse Events
Only 1 patient (3.2%) in BG and 3 in CG (9.7%) suffered from nausea, of which 2 patients in CG needed treatment. Two patients (1 in each group) experienced shoulder pain. No vomiting was recorded. However, all patients reported comfort throughout the procedure and did not experience any side effects determined to be directly related to the ESPB.
5. Discussion
This study suggests that ESPB is an effective analgesic strategy that is easy to perform, especially in the outpatient setting (44-47).
The results of this study are consistent with prior data and support the efficacy of ESPB for visceral pain control. For instance, in a case series, Chin et al. showed that ESPB could effectively block the visceral pain associated with bariatric surgeries (40); Also, other studies on the effect of ESPB on post-LC (4, 32-37) proved the fact that most of the source of the post-LC pain has a visceral origin (12). However, all studies on ESPB for post-LC analgesia have used a bilateral approach (4, 32-37). To the best of our knowledge, this is the first report of the proven efficacy of unilateral ESPB for post-LC pain control in a content of a randomized control trial with a larger sample size.
The mechanism of somatic and visceral analgesia of ESPB has been a central focus of research in this field. There are multiple human cadaveric studies with considerable discrepancies among the results (48). The number of cadaveric investigations of the spread of stained local anesthetic to neural foramina, paravertebral, and/or epidural space (49-51) is almost equal to those which failed to show that (52-54). Although ESPB has been performed unilaterally in these cadaveric studies, spread to the contralateral side of injection has not been reported. Meanwhile, a growing body of evidence supports or rejects if the truncal fascia plan block, including ESPB, can be used as a regional anesthesia modality to control visceral pain (55).
A case report of 3D computed tomography scan images on patients with T5 ESPB demonstrated the spread of the contrast to costotransverse foramen at the level of the T6-T0. Thus, costotransverse foramen could be the possible gate of the reach of local anesthetics during ESPB to more ventral neural structures (56). Using magnetic resonance imaging of the spine and injection of the 30 mL of the mixture of local anesthetic and gadolinium contrast at T10, Schwartzmann et al. showed circumferential T5 to T12 epidural and paravertebral spread through the left T5 to T12 intervertebral foramina after a T10 left-sided ESPB (41). This finding may explain the bilateral sensory changes that Tulgar et al. mentioned (42). These results, along with studies on the spread to ventral rami of the intercostal nerves in the paravertebral space (14), rami communicants, and sympathetic chain (57), may explain the visceral pathway blockade (at the intervertebral foramen where the greater and lesser splanchnic nerves merge) and the bilateral multiple spinal segmental blockades through the circumferential epidural spread.
Mechanisms of the bilateral sensory changes after a unilateral single injection of ESPB are also most likely related to the spread to the epidural space and the contralateral sensory block.
However, a possible alternative explanation for this observation might be the contralateral passage through the interspinous ligament as observed with the retrolaminar block (58), especially if the injection is closer to the lamina than the tip of the TP.
Many postulating factors that can result in a bilateral spread after unilateral block include the volume and concentration of the local anesthetics, injection closer to the lamina (resulting in retrolaminar spread), and the structural variations of the patient’s erector spinae muscles and fascia planes. In addition, the type of surgery and patient positioning may contribute to the spread to the contralateral side. More anatomical and radiological studies may thus be needed to explain the bilateral sensorial block caused by unilateral ESPB.
It is essential to emphasize that innervation of the intra-abdominal viscera is complex and involves bilateral sympathetic and parasympathetic nervous systems. Innervation of the gallbladder and liver involves celiac ganglion, superior mesenteric ganglion, prevertebral, and paravertebral ganglia using greater and lesser splanchnic nerves, as well as white rami communicants to the dorsal root ganglion and the spinal cord. A blockade on this pathway is the key to providing visceral analgesia for a successful ESPB, either unilateral or bilateral. More studies on live subjects may reveal the site of action of ESPB on the visceral track (59, 60). In this study, we were able to leverage ropivacaine use with lower cardiotoxicity. It should be noted that performing unilateral ESPB will reduce the time of the block, reduce the total amount of local anesthetic dosage and the risk of toxicity, and have a similar analgesic efficacy as bilateral ESPB for post-LC pain control. Postoperative analgesia at rest and during cough extended up to 24 hours, which may indicate the duration of ESPB in this group of patients.
However, more studies are needed to fully elucidate this result.
Our study has its limitations. This research study occurred at a single center with a small sample size. Though we found a difference, it is possible that this may not be generalizable to other hospital settings or institutions. This research was also single-blinded, and patients in CG did not get a sham block. Therefore, patients in BG might report less pain because of the awareness of ESPB. However, the difference in narcotic usage, either as PCIA or rescue meperidine, was so significant, indicating that blinding alone may not explain this difference. Performing a double-blinded cross-over randomized control trial may overcome some of these limitations. Our pain measurements were limited to the first 24 hours post-PACU. It is possible that some shoulder pain associated with LC may have been missed, as it is more prominent after 24 hours. The long-term effect of having or not having ESPB in LC was not considered in this study. Non-steroidal anti-inflammatory drugs were not part of the multimodal analgesia in this study, as it was not a standard of care in that facility. Of note, however, if we had applied a conservative post hoc test (eg, Bonferroni adjustment with α = 0.05/14 [7 assessments of pain at rest and 7 with cough] = 0.004), our associations would retain significance; therefore, we believe that these interpretations are robust. Finally, we did not assess the impact of the unilateral ESPB on gastrointestinal function, discharge, or overall cost of care. More studies are needed to confirm these outcomes. A preoperative block might also reduce or eliminate intraoperative narcotic usage, another indicator of a successful block.
5.1. Conclusions
Performing right-sided unilateral ESPB at the level of T7 is effective pain management for post-LC. It reduces acute postoperative pain and the need for narcotic analgesia and may improve patient satisfaction.
5.2. What Is Known
• Post-LC pain is multifactorial; thus, multimodal analgesia has been suggested for its treatment.
• Visceral pain has been the primary source of postoperative pain in LC, especially in the first 24 hours.
5.3. What Is New
• Performing right-sided unilateral ESPB at the level of T7 is effective pain management for post-LC.
• Right-sided unilateral ESPB at the level of T7 reduces the acute postoperative pain and the need for narcotic analgesia and may improve patient satisfaction.