1. Background
Globally, approximately 310 million surgeries are performed yearly, with many patients requiring endotracheal intubation to successfully conduct these procedures (1). Laryngoscopy, tracheal intubation, surgical stimulation, and extubation incite remarkable sympathetic activity and are associated with transient but significant hemodynamic changes, termed as pressor/hemodynamic response, characterized by a sudden surge in mean arterial pressure (MAP) and heart rate (HR), arising 30 s following direct laryngoscopy and endotracheal intubation (LI), approaching baseline in 10 minutes (2, 3). This short-lived exaggerated response may precipitate hypertensive episodes, cardiac arrhythmias/ischemia, or intracranial hypertension in susceptible individuals (4). The need to blunt these noxious responses effectively may lead to using several techniques and pharmacological agents, local anesthetics, beta-adrenergic-blockers, calcium channel antagonists, and opioids with varied success (2, 4). Intravenously (IV) administered selective alpha-2 agonists are known to obtund LI-associated sympathoadrenal responses (3).
However, a few unfavorable features being reported with concerns like delayed recovery (owing to its sedative actions), hypotension, bradycardia, and rarely cardiac arrest often hamper the widespread use of IV dexmedetomidine (IV-Dex); hence, a lookout for alternative modes of dexmedetomidine administration continues. Alternative modes of dexmedetomidine administration are expected to limit the adverse effects and enhance the safety profile of dexmedetomidine (2). The efficacy of intranasal dexmedetomidine has been investigated with established safety, efficacy, and high patient acceptance. However, occasionally intranasal dexmedetomidine may be associated with nasal irritation. Nebulized dexmedetomidine (Neb-Dex) may offer a viable alternative, owing to drug deposition over a greater surface area (nasal, buccal, and respiratory mucosa), resulting in better systemic absorption and prevention of postoperative sore throat in addition to achieving the attenuation of laryngoscopy response, sedation, and analgesia effects (2, 4). This research was undertaken owing to the paucity of medical literature comparing the efficacy of IV-Dex and Neb-Dex in equivalent doses.
2. Objectives
The primary objectives were to evaluate and analyze hemodynamic sequelae of preoperative IV-Dex and Neb-Dex during LI and compare their efficacy in blunting the sympathoadrenal response. The secondary objectives were to evaluate intraoperative analgesic consumption, hemodynamics, and postoperative sore throat (POST) incidence.
3. Methods
This prospective, single-centric, double-blinded, randomized, Helsinki protocol-compliant clinical study was conducted after obtaining written informed consent and approval of the institutional ethics committee (SRMSIMS/ECC/2021-22/062) and registration with the Clinical Trials Registry of India (CTRI) (CTRI/2022/07/043841).
We enrolled 120 ASA PS I/II, aged 18 - 60 years, undergoing elective short-duration surgical procedures under general anesthesia. Patient refusal, dexmedetomidine allergy, preoperative sore throat/upper respiratory tract infection, significant cardiac-pulmonary/hepatorenal disease, body mass index (BMI) > 30 kg/m2, predicted difficult airway/unanticipated difficult intubation or laryngoscopic attempt lasting greater than 15 s or two attempts or more were the exclusion criteria. Those with a history of chronic opioid use/opioid addiction, steroids, emergent surgeries, parturients, and those developing any intra-/postoperative complication or extended surgery duration were also excluded.
Enrolment of patients commenced in July 2022 and was completed in September 2022. Patients were randomly assigned into two groups by a computer-formulated randomization technique. The method of concealment was consecutively numbered opaque sealed envelopes. Study subjects were randomized between groups 1 and 2 with 60 subjects each: Group 1: IV-Dex (1 mcg/kg body weight administered over 10 min) and Group 2: Neb-Dex (1 mcg/kg body weight diluted with normal saline 4 ml administered over 10 min).
A detailed pre-anesthetic check-up was conducted a day prior to the elective surgery. All patients’ demographic data (age, weight, height, and BMI) were recorded. During the preoperative visit, all patients were familiarized with POST scale (5): (1) 0, no sore throat; (2) 1, mild sore throat (reporting sore throat when enquired); (3) 2, moderate sore throat (reports without asking); and (4) 3, severe sore throat (voice change/hoarseness/pain in the throat). Lukewarm saline gargle and decongestants were prescribed for sore throat persisting after 24 h. An otorhinolaryngology (ENT) consultation was ensured in case of a persistent/severe throat complaint.
All standard monitors were applied. In both groups, allotted preparation was administered 30 min before anesthesia induction. After premedication (IV glycopyrrolate 4 mcg/kg and midazolam 0.03 mg/kg), 2 mcg/kg IV fentanyl was administered. Anesthetic induction commenced with propofol till the loss of verbal command. Endotracheal intubation (ETI) was facilitated with vecuronium (0.1 mg/kg). Pre-emptive IV Paracetamol 1000 mg was administered 10 min following ETI. Escalation in MAP (> 20% baseline) in the 10 min interval following LI was treated with aliquots of propofol (20 - 30 mg IV). Injectionesmolol 10 mg boluses were reserved as a rescue drug for those not responding to propofol aliquots, whereas the boluses of injectable mephentermine 6 mg and 0.6 mg of atropine IV were used to address MAP, and HR decreases, respectively. No intervention was allowed till 10 min after intubation. Maintenance of anesthesia was done using an oxygen/nitrous-oxide/isoflurane mixture to maintain the Bispectral index at 40 - 60 and neuromuscular monitoring guided vecuronium boluses. The intraoperative analgesic requirement was recorded. Residual neuromuscular blockade was reversed by administering IV neostigmine 0.05 mg/kg + inj. glycopyrrolate 0.005 mg/kg. Patients were transferred to the postanesthesia intensive care unit (PACU) after extubation. The hospital pain management protocol was followed thereafter. The HR and MAP were noted at baseline (TBl), after nebulization (TN), after induction (TInd), immediately following intubation (TInt), at 1 min (T1), 3 min (T3), 5 min (T5), 7 min (T7), 10 min (T10), 15 min (T15), 30 min (T30), 45 min (T45), 60 min (T60), 75 min (T75), 90 min (T90), 105 min (T105), and 120 min (T120). Analgesia was supplemented by 0.5 mcg/kg of fentanyl, and esmolol 10 mg boluses were reserved as a rescue drug for a rise in HR or MAP, after an initial 10 min, and 20% above baseline intraoperatively.
Duration of surgery (from skin incision to completion of skin closure) was noted. The total duration of anesthesia from the time of anesthesia induction to tracheal extubation was calculated in minutes (min). Time to awakening from the end of skin closure to patient extubation was also noted.
Postoperative pulse oximetry, heart rate, mean blood pressure, “Richmond Agitation Sedation Score (RASS)” monitoring, and POST were evaluated 0, 1, 2, 4, 6, 8, 12, and 24 h post-extubation.
3.1. Statistical analysis
The sample size was calculated based on Misra et al. (4). Fifty patients in each group were necessary to power the study to 80% to detect the difference with an alpha error of 5%, presuming a 20% difference in the highest HR between the two groups after laryngoscopy (two-tailed). The standard deviation of the pool was 35. We recruited 120 patients, accounting for 20% dropout after recruitment (unanticipated difficult intubation, laryngoscopy taking more than 15 s or two tries, or protocol violation). SPSS version 28.0 software for Windows was used to conduct the statistical analysis. Continuous variables are presented as mean ± SD, median (IQR), and minimum-maximum values. Categorical variables are displayed as percentages and absolute values. Prior to statistical analysis, the normality of the data was verified. The Mann-Whitney U test was employed for variables that were not normally distributed, whereas the unpaired t test was used to compare continuous variables that were normally distributed. The Fisher’s exact or chi-square tests were used to assess categorical variables.
For within-group comparisons, a paired t test was used to test any significant change in hemodynamic parameters at various time points from the baseline. The P values less than 0.05 were used to denote a significant difference for all statistical tests.
4. Results
The CONSORT flow diagram (Figure 1) depicts the flow of the participants in the three groups. Demographic variables revealed that both groups were comparable (Table 1).
| Parameters | Group 1 | Group 2 | P Value | ||||
|---|---|---|---|---|---|---|---|
| Mean ± SD | Min - Max | Median (Q1-Q3) | Mean ± SD | Min - Max | Median (Q1-Q3) | ||
| Sex Distribution | 0.7434 | ||||||
| Male | 32 | 30 | |||||
| Female | 28 | 30 | |||||
| ASA physical class | 0.702 | ||||||
| I | 22 | 20 | |||||
| II | 28 | 40 | |||||
| Age | 42.3 ± 10.761 | 18 - 60 | 43 (34.5 - 51.75) | 41.1 ± 10.006 | 19 - 62 | 41.5 (35 - 47.5) | 0.528 |
| Body mass index | 25.53 ± 4.483 | 15 - 37 | 25 (22 - 28.675) | 24.53 ± 2.021 | 20 - 32 | 25 (23 - 26) | 0.120 |
Additionally, the two groups were similar in the duration of surgery/anesthesia, the initial bolus of dexmedetomidine, fentanyl, and propofol, and the intra-operative fentanyl rescue dose (Table 2).
| Variables | Group 1 (n = 60) | Group 2 (n = 60) | P Value | ||||
|---|---|---|---|---|---|---|---|
| Mean ± SD | Min - Max | Median (Q1-Q3) | Mean ± SD | Min - Max | Median (Q1-Q3) | ||
| Duration of surgery | 84.55 ± 15.887 | 40 - 108 | 90 (72.75 - 96) | 88.9 ± 13.111 | 47 - 106 | 90 (85.25 -99.75) | 0.216 |
| Duration of anesthesia | 104.65 ± 14.463 | 63 - 140 | 105.5 (93.25-117.75) | 106.33 ± 13.924 | 60 - 120 | 105 (103.25 - 120) | 0.491 |
| Dexmedetomidine dose | 61.2 ± 9.756 | 34 - 82 | 60 (54.25 - 68) | 62.23 ± 8.028 | 48 - 84 | 62 (56 - 67.5) | 0.651 |
| Propofol | 122.9 ± 18.709 | 70 - 160 | 120 (110 - 140) | 121 ± 12.716 | 100 - 140 | 120 (120 - 130) | 0.444 |
| Fentanyl bolus | 119.57 ± 22.551 | 68 - 185 | 120 (100 - 130) | 121 ± 12.716 | 100 - 140 | 120 (120 - 130) | 0.404 |
| Fentanyl (intraoperative) | 26.75 ± 14.81 | 0 - 70 | 30 (25 - 35) | 27.67 ± 12.054 | 0 - 50 | 30 (25 - 35) | 0.865 |
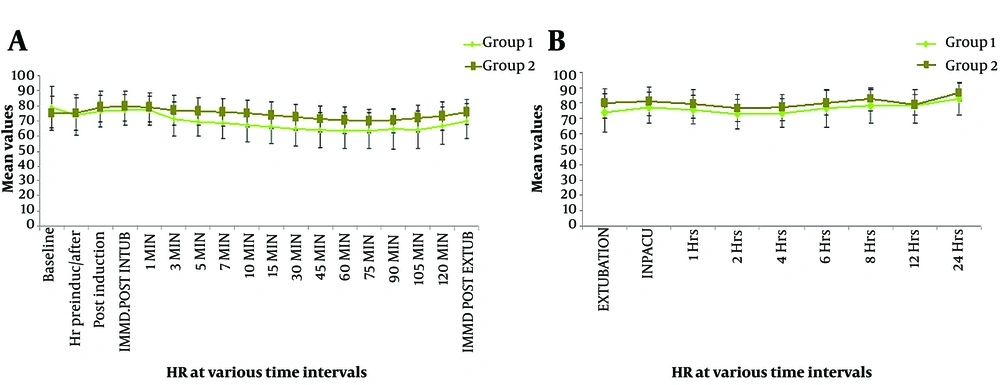
Primary outcome measures were changes in HR and MAP upon LI compared to baseline at the given time points. At TB, there was no significant difference in the mean HR (P = 0.081), TN (P = 0.706), TInd (P = 0.136), TInt (P = 0.138), and T1 (P = 0.194). Subsequently, a significant difference was observed in the mean HR (P < 0.05). Initial monitoring for up to 10 minutes was performed to assess LI response, while subsequent monitoring was done to assess secondary objectives. Intragroup variability in HR from baseline at different time points was analyzed in terms of percentage difference. Compared to baseline, a serial fall in HR was observed in the IV-Dex group (-6.5%, -3.5%, -2.6%, -7.2%, -10.2%, -12.1%, -13.7%, -16.9%, -18.2%, -19.5%, -19.9%, -20.2%, -19.0%, -20.8%, -16.8%, and -11.5%). In contrast, the corresponding values in the nebulized dexmedetomidine group showed modest increases in HR (maximum at immediate post-intubation and 1 min post-intubation, maximum of +6.3%), with values returning to baseline at 10 minutes post-intubation. Percentage variations in HR compared to baseline in group 2 at all time points were -0.3%, +5.4%, +6.3%, +5.4%, +3.0%, +1.8%, +0.9%, -0.4%, -1.7%, -3.5%, -5.2%, -6.3%, -6.8%, -5.9%, -4.0%, -1.4%, and +1% (Figure 2).
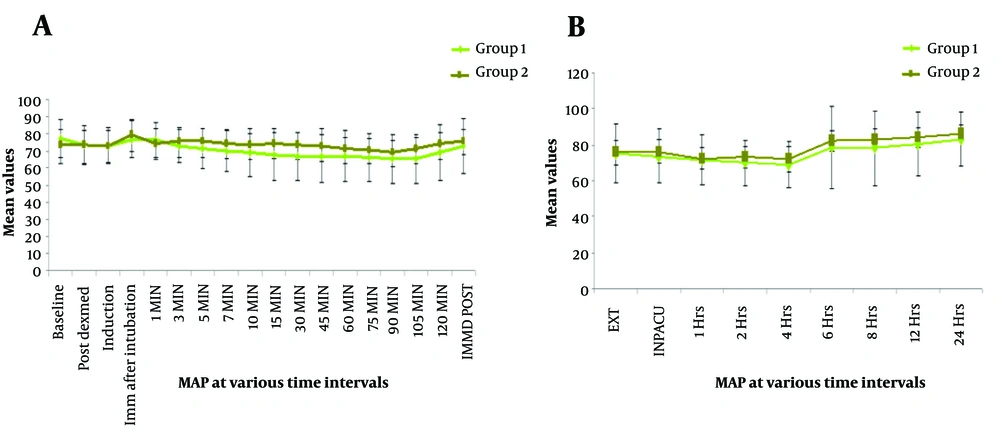
Similar trends were also observed in terms of MAP. It was noted that the mean MAP did not significantly differ from one another at baseline (P = 0.070), post dexmedetomidine (P = 0.971), post-induction (P = 0.977), immediately after intubation (P = 0.095), and at 1 min (P = 0.291). A significant difference between the two groups’ MAP levels was seen at most stages up until the end of the surgery. Intragroup deviation in MAP at different time points was analyzed in terms of percentage variability compared to baseline. Compared to baseline, a serial fall in MAP was observed in the IV-Dex group (-4.9%, -5.2%, -0.4%, -0.9%, -5.8%, -7.2%, -8.9%, -10%, -11.8%, -13.2%, -12.9% -13.3%, -13.1%, -13.4%, -13.6%, -11.9%, and -5.5%). When compared to the Neb-Dex group’s equivalent values, there was a slight increase in MAP (peaking immediately post-intubation), with values reverting at 10 minutes. Percentage variations in MAP compared to baseline in group 2 at all time points were -0.47%, -1.0%, +8.0%, +1.2%, +3.0%, +2.0%, +0.8%, -0.4%, -0.6%, -0.1%, -1.6%, -3.2%, -4.2%, -6.1%, -4.2%, -0.1%, and +1%. At no time point did the rate pressure product reach a critical ischemia value of 12,000 in either group. Blood oxygen saturation levels were insignificant between the two groups at all time points (Figure 3).
In the postoperative period, recovery/sedation was assessed through the RASS. Mean RASS was compared between the two groups postoperatively at pre-defined time points. A significant difference existed in mean RASS at extubation, in PACU, and at 1 h postoperatively (P < 0.001). Subsequently, the two groups had no significant difference in sedation levels. POST was evaluated during the same intervals following surgery. There was a significant difference in the mean POST postoperatively with significantly lower sore throat incidence in the Neb-Dex group. The side effect profile exhibited a higher number of adverse events in the IV-Dex group though the difference was insignificant (Table 3).
| Variables | Group 1 (n = 60) | Group 2 (n = 60) | P Value |
|---|---|---|---|
| Bradycardia | 4 (6.7) | 0 (0.0) | 0.119 |
| Hypotension | 4 (6.7) | 0 (0.0) | 0.119 |
| Nausea/vomiting | 0 (0.0) | 0 (0.0) | |
| Other | 0 (0.0) | 2 (3.3) | 0.496 |
a Values are expressed as No. (%).
5. Discussion
General endotracheal anesthesia is a common anesthesia modality employed worldwide, as it helps attain a state of balanced anesthesia while ensuring adequate airway protection. An anesthesiologist’s major challenge is the attenuation of LI response, which is a centrally mediated sympathetic reflex secondary to stretching the laryngeal and pharyngeal tissue (6). This phenomenon was first described by Reid and Brace in 1940 (7). Alpha-2 agonists (IV dexmedetomidine/clonidine) have shown promise not only for LI response attenuation but also as a sedative, anxiolytic and analgesic. Rapid IV administration has a propensity for bradycardia, hypotension, and a biphasic action (2). Despite a proposed antidote that increases the central noradrenaline turnover, its cost and availability make its usage less frequent (2, 8). A constant lookout for other routes (oral/intramuscular/intranasal and recently nebulized) continues to offer an alternative with fewer side effects. The intranasal (IN) route has been explored; however, IN administration requires a specific atomization device, the availability of which may prove a hindrance. IN-dexmedetomidine crosses the blood-brain barrier easily, as dexmedetomidine is lipophilic, which, coupled with high vascularity of nasal and laryngotracheal mucosa helps dexmedetomidine bypass first-pass metabolism (9-11). This may well apply to the nebulized route, which augments the surface area coverage with a thin layer of the drug (12). We attempted to investigate Neb-Dex and compare its efficacy with IV-Dex for attenuating LI since literature regarding Neb-Dex is primarily restricted to procedural sedation as premedication or helping allay separation anxiety in pediatric age group (13-18). Nebulization provides advantages including cheaper and easier administration, utilization even in resource-limited settings (not requiring special devices like syringe pumps/intranasal atomization devices, etc.), homogenous deposition of the drug in the nasal/pharyngeal tract, improving expiratory mechanics in the setting of asthma/COPD by nebulized alpha-2 agonists and potential role of Neb-Dex in sedation/attenuation of POST, thereby making the nebulized route potentially more holistic (5, 19).
Direct LI can increase the HR and MAP by 20 - 27% and 30 - 50%, respectively (20). Our study revealed no significant difference in hemodynamics (HR/MAP) between the two routes at TBl, TN, TInd, TInt, and T1. In both groups, the hemodynamic parameters following laryngoscopy-intubation were within normal limits (+20% of baseline). Maximum HR in the IV-Dex group was 2.6% lower than the baseline, while in the nebulized group, it was 6.3% higher. Maximum MAP in the IV-Dex group was 0.4% lower than baseline and 8% above baseline in the Neb-Dex group. These variations were within the accepted definitions of hemodynamic changes following the LI response. Also, the intergroup difference was statistically insignificant. The lowest HR in the IV-Dex group was 20% of the baseline, while in the Neb-Dex group, it was 7%. Along the same lines, in terms of MAP, the lowest value was 13% of the baseline, and the corresponding value in the Neb-Dex group was 6.8%. Hemodynamics in the nebulized group returned to the baseline within 10 minutes following intubation. The significant difference in the hemodynamics (HR and MAP) post 3 min was primarily due to greater intra-group fall in HR and MAP in the IV-Dex group compared to the Neb-Dex group and the intra-group hemodynamics in the nebulized group experiencing more resistance to hypo/hypertension and Brady/tachycardia with a lesser and slower rate of hemodynamic changes. At no time did the rate-pressure product reach the critical ischemia value of 12000 in either group, making Neb-Dex a potential alternative for patients who are poor candidates for hypo-/hypertension or Brady/tachycardia. Our results were partly in line with Misra et al. (4), who reported that nebulized dexmedetomidine attenuated the ascent of HR but failed to arrest MAP rise. These findings may be explained based on the fact that the bioavailability of dexmedetomidine through the nasal and buccal mucosa is 65% and 82%, respectively, making 1 mcg/kg nebulized dose equivalent to 0.5 mcg/kg. The bioavailability of dexmedetomidine by other non-intravenous routes is orogastric (16%) and intramuscular (104%) (14). Dexmedetomidine adverse effects are dose-dependent, prompting us to analyze equivalent doses restricted to 1 mcg/kg. Another study involving the effect of nebulized dexmedetomidine on the attenuation of LI response by Kumar et al. indicated the efficacy of the nebulized route, which is similar to ours (21). However, both studies compared the nebulized route with saline nebulization and did not compare the efficacy of the nebulized route with other routes (IV/Intranasal). This was considered a limitation by Misra et al. and has been addressed by us. Our study not only dealt with this lacuna but also compared intra-operative analgesic consumption, hemodynamics, and POST as secondary endpoints, which have not been assessed by most studies (4).
The propofol and intra-operative fentanyl consumption was equivalent in both groups, indicating good efficacy in reducing anesthetic drugs requirement (hypnotic/sedative/analgesic) via the nebulized route akin to IV. Our findings corroborated with Misra et al. (4), Kumar et al. (21), and Shrivastava et al. (22). In the postoperative period, recovery was assessed in terms of sedation using the RASS. In our study, significant sedation was observed till one hour postoperatively in the IV-Dex group. The intra-operative analgesic requirement is reduced due to the alpha-2 adrenergic receptor agonist activity at spinal and supra-spinal levels (dorsal horn and locus coeruleus), causing analgesic and sympatholytic activity of dexmedetomidine (23). This can probably be due to the elimination half-life of IV dexmedetomidine in healthy adults being 2.1 - 3.1 h (24, 25). In trials by Misra et al. (4), Kumar et al. (21), and Shrivastava et al. (22), Neb-Dex did not cause significant sedation. Even though dexmedetomidine-induced sedation resembles normal, arousable sleep and is termed cooperative sedation, the authors believe this can be a cause of caution in patients who are poor candidates for postoperative sedation (obstructive sleep apnoea COPD, etc.) and in conditions where postoperative monitoring facilities are not up to acceptable standards, especially in resource-limited settings.
POST is regarded as the “big-small problem” in the postoperative phase and has a 21 - 65% incidence (4). POST was assessed in all study subjects. Inter-group comparison of POST at pre-defined time points indicated a significant difference with significantly lower sore throat in the Neb-Dex group. IV-dexmedetomidine, owing to its tendency to lower inflammatory markers (TNFα, IL-6, and S100β), has favorable effects on POST (26, 27). Thomas et al. (5) and Jandial and Tabassum (28) investigated the role of Neb-Dex on POST in thyroidectomy patients and reported a favorable outcome. Both Neb-Dex and IV-Dex groups exhibited a favorable effect on POST, with Neb-Dex performing better. The reason for this needs to be investigated as to whether Neb-Dex exerts any local/topical effects on laryngotracheal mucosa. Misra et al. did not report any significantly favorable outcome on POST by Neb-Dex (4).
In terms of adverse effects, the difference between the two groups was non-significant; but the IV-Dex group had 4/60 patients, each exhibiting bradycardia and hypotension, who responded to corrective measures. No patient experienced PONV. The same was demonstrated by investigations involving Neb-Dex. This proves the equivalent efficacy of nebulized dexmedetomidine in preventing PONV and can be attributed to opioid-sparing, alpha-2 receptor agonistic action, and sympatholytic effects.
5.1. Limitation
No study, including ours, is devoid of any limitations. We evaluated a single dose of dexmedetomidine, and higher doses via both intravenous and nebulized routes need to be investigated. Also, no study has to date compared the nebulized versus intranasal route. This was also a limitation in our research, and a three-pronged comparison between the IV, nebulized, and intranasal routes should be conducted. Even though the sample size was based on previous studies, a larger study is needed to generalize the results and find further explanations for unexplained findings.
5.2. Conclusions
A single 1 mcg/kg dose of nebulized dexmedetomidine 30 minutes prior to induction blunts the LI response and reduces intra-operative analgesic consumption. Nebulized dexmedetomidine may provide a favorable alternative to the IV route in patients who are poor candidates for tolerating hypotension, bradycardia, and postoperative sedation undergoing short-duration surgeries without any significant side effects.