1. Background
Osteoarthritis (OA) is the most frequent degenerative joint problem worldwide (1). It is more common among the elderly, with 10% and 18% among male and female elders, respectively (2, 3). This slow, progressive degenerative disease might cause chronic pain, joint damage, depression, and social isolation. Pain and loss of function are the major reasons for functional dysfunction, decreased quality of life (QoL), and severe socioeconomic consequences for society in developed countries (4-6).
Osteoarthritis mostly occurs in the knee, hip, hand, spine, and foot joints, with less frequent development in the wrists, shoulders, and ankles. Joint pain is the major symptom of OA (3, 6), and almost 50% of patients with knee pain demonstrate radiographic alterations and, therefore, will be classified as symptomatic OA. Nevertheless, the relationship between joint pain and the radiographic properties of OA is volatile (7).
Osteoarthritis and consequent pain in large weight-bearing joints often lead to major functional andphysical problems (8, 9) and might require invasive surgical interventions (10). General treatment has been focused on pain management and improvement of joint function; meanwhile, enhanced awareness about OA pathophysiology inspired the introduction of disease-modifying osteoarthritis drugs (DMOADs), such as matrix metalloproteinase inhibitors, bisphosphonates, cytokines, calcitonin, and nitric oxide synthase inhibitors (11, 12).
Currently available therapeutic options for OA range from pharmacological and physical interventions to the intra-articular administration of steroids or hyaluronic acid. Prolotherapy and Prolozone are other new treatments for OA (13, 14). In a study that aimed to compare the impact of periarticular and intra-articular prolotherapy on the pain and disability of individuals suffering from knee OA, Rezasoltani et al. reported similar effects with no consequence (14, 15).
Calcitonin has long been used to treat osteoporosis and has recently been studied as a potential therapeutic option for OA (16), according to its association with the cartilage parts of the joint, in terms of metabolic functions (17). Calcitonin is a 32-amino-acid peptide that contains anti-resorptive properties. Several studies mentioned its positive impacts on chondrocytes and osteoclasts (18). Preclinical models indicated its ability to prevent OA progression. According to the literature, calcitonin can have anti-catabolic properties, mainly through declining proteoglycan and collagen type II degradation by stimulating their production (17). Due to the analgesic and beneficial effects of calcitonin on cartilage and subchondral bone, it has been hypothesized that it might be considered a potential therapeutic option in OA. Therefore, this study aimed to assess the efficacy of intramuscular calcitonin injection on the functional status of patients suffering from knee OA.
2. Objectives
This study was designed to assess the efficacy of the intramuscular injection of calcitonin on the functional status of patients who suffer from knee OA.
3. Methods
Following a randomized controlled trial, this study was performed in an academic referral center within August to November 2020. All related national principles and those mentioned by the Declaration of Helsinki were observed. In addition, the current study was approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences, Tehran, Iran (ethical approval no.: IR.SBMU.RETECH.REC.1399.362). All the patients were included after signing a written informed consent form. The clinical trial number for this study is IRCT20130518013364N9.
The study population consisted of patients of both genders with OA of at least one knee who were referred to the Outpatient Pain Clinic in Imam Hossein hospital, Tehran, Iran, within 4 months (August-November 2020). The inclusion criteria were an age range of 35 - 75 years, knee pain during the past 6 months, and at least two clinical symptoms of joint inflammation based on the American College of Rheumatology indicators for OA (both clinical and clinical plus radiographic) (Box 1). The American College of Rheumatology has published a set of clinical classification indicators to categorize knee OA, which are popular and are used by several studies (19).
| Clinical and Radiographic Classifications |
|---|
| The patient had joint pain on most days of the previous month. |
| 1. Crepitus during active joint movement |
| 2. Duration of morning stiffness less than 30 minutes |
| 3. Age over 38 years |
| 4. Knee bone enlargement during the examination |
| 5. Knee bone tenderness during the examination |
| 6. Absence of palpable pulse |
Acceptable Criteria for Clinical Classification of American College of Rheumatology and Clinical Radiographic Classification of Knee Osteoarthritis
The exclusion criteria included unwillingness to participate in the study, a history of cardiovascular, renal, hepatic, or gastrointestinal diseases, a history of coagulopathy, currently using DMOAD agents (e.g., hydroxychloroquine, sulfasalazine, methotrexate, cyclosporine, and azathioprine), pre-existing allergies to calcitonin, a history of intra-articular injection into the knee in 3 months before participating in the present study, having already received total knee arthroplasty, a recent history of substance abuse, and inability to reliably report outcome measures due to cognitive impairment.
The sample size was determined to be 40 (two groups of 20), using a two-sample mean comparison test assuming an α-error of 0.05, power of 80%, and drop-out rate of 20%. Sampling was performed based on the census method, and all eligible patients were included until the above-mentioned sample size was reached.
On admission (baseline), demographics and baseline data, including pain intensity using the Numeric Rating scale (NRS) and Persian-validated self-administered Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) questionnaire, were collected for all eligible patients. The data were entered into patients’ profiles by the principal investigators. Care was taken for each patient to be adequately explained about the study’s aims and objectives and the correct method of responding to the questions.
The Numeric Rating scale is a frequently applied self-report scale intended to measure the pain score, ranging from 0 (no pain) to 10 (the highest imaginable pain) (20). The Western Ontario and McMaster Universities Arthritis Index is the most frequently applied disease-specific measure for measuring the dimensions of pain, dysfunction, and joint stiffness in those who suffer from knee and hip OA, with 25 items that are categorized into three groups, including pain (6 items), stiffness (2 items), and physical movement (17 items). Patients should respond to each question on a scale of 0 (none) to 4 (extreme) (21).
The patients were randomly allocated to either group of cases or controls using the simple randomization technique. Both groups were prescribed with AcetaGel (500 mg) and piroxicam (0.5% topical gel) every 8 hours as needed. The patients were also instructed about conservative treatments and lifestyle modifications. In the case group, the patients received calcitonin (50 IU/mL solution for injection; Aburaihan Pharmaceutical Co., Iran) intramuscularly (gluteal muscle) once a week for 4 consecutive weeks. The evaluation was repeated 2 months after the first evaluation (1 month after the fourth injection in the case group) using the WOMAC questionnaire. At this stage, the physician evaluating the patient was not aware of the patient’s group (i.e., cases or controls).
The data were analyzed by SPSS software (version 21). A two-sample t-test and repeated measurements analysis of variance were applied to compare the data at the baseline and 1 month after the last medication. A p-value less than 0.05 was considered statistically significant.
4. Results
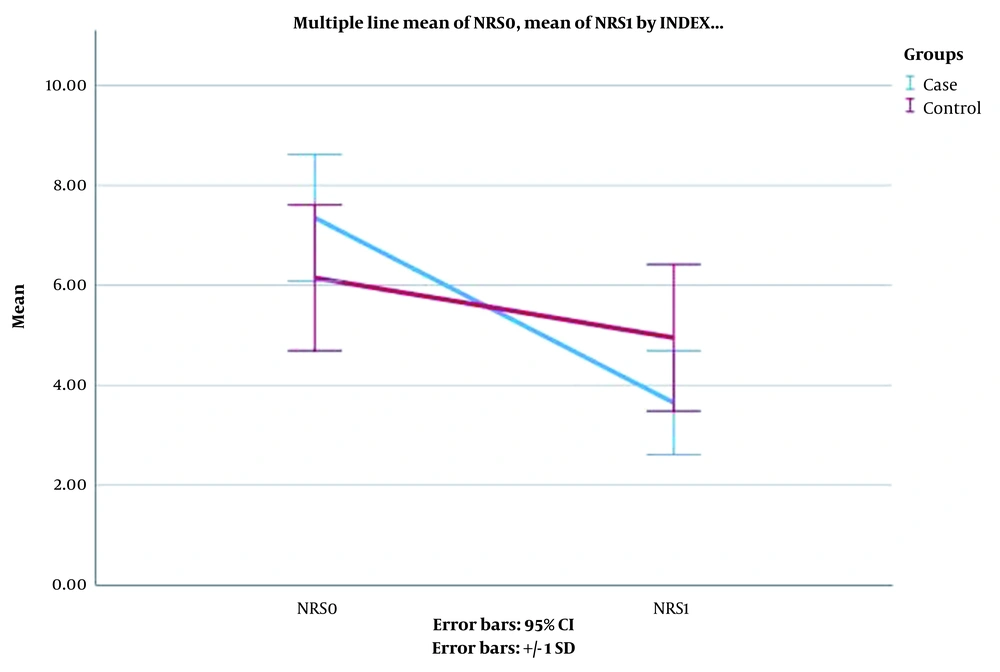
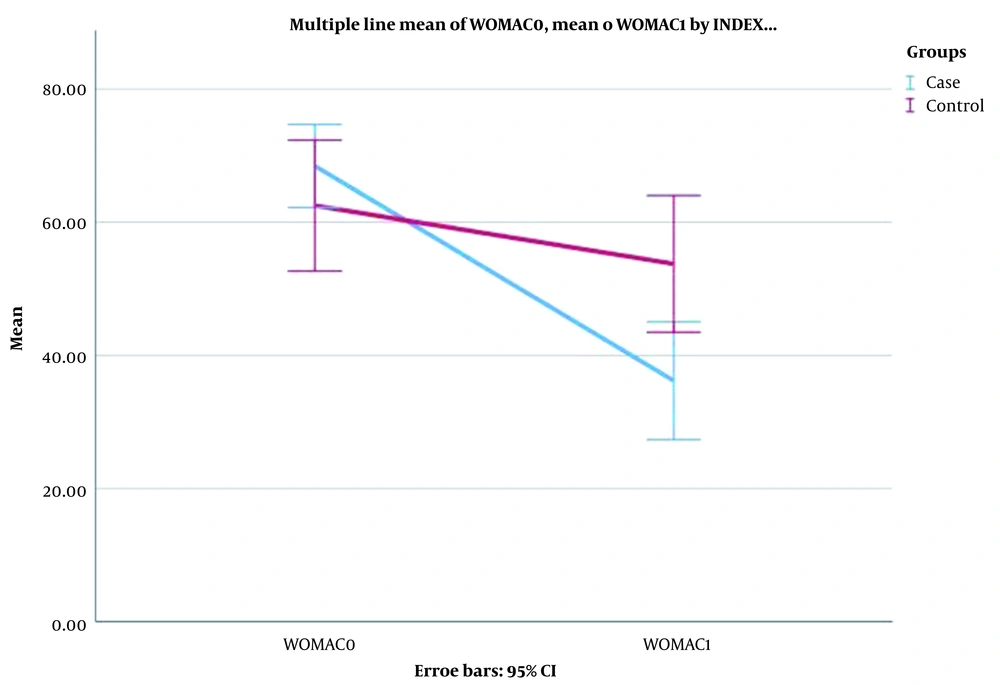
A total of 40 cases completed the study. The gender, age, and body mass index of the participants did not show any statistically significant difference. Table 1 shows the demographic data. After a month, significant improvements were observed in WOMAC and NRS outcomes. The patients reported a significant reduction in NRS scores one month after treatment with four injections of 50 mg calcitonin (P < 0.001). Table 2 shows the obtained data in this regard. Figures 1 and 2 illustrate the comparison of NRS and WOMAC scores in both groups at the baseline and after the treatment.
| Case (n = 20) | Control (n = 20) | P-Value | |
|---|---|---|---|
| NRS | |||
| Baseline | 7.35 ± 1.27 | 6.15 ± 1.46 | 0.009 |
| After intervention | 3.65 ± 1.04 | 4.95 ± 1.47 | 0.004 |
| Difference | 3.70 ± 1.08 | 1.20 ± 0.69 | < 0.001 |
| WOMAC | |||
| Baseline | 68.45 ± 6.24 | 62.50 ± 9.82 | 0.043 |
| After intervention | 36.20 ± 8.85 | 53.75 ± 10.27 | < 0.001 |
| Difference | 32.25 ± 9.23 | 8.85 ± 4.40 | < 0.001 |
Baseline Characteristics, Western Ontario and McMaster Universities Osteoarthritis Index, and Numeric Rating Scale Scores after One Month of Calcitonin Treatment a
5. Discussion
This study was designed to assess the efficacy of calcitonin in the pain and functional ability of patients suffering from knee OA. Due to the high prevalence of OA worldwide (1), this disease is one of the main concerns of physicians, researchers, and socioeconomic officials with a great disease burden over the country (4). Based on a recent World Health Organization report, the disease will be the fourth leading cause of physical disability in the world by 2020. Based on this report, globally, 28% of those over 65 years suffer from the disease, with an annual cost of 4,800 million € to the healthcare system (3, 5). Therefore, due to the insignificant effects of the drugs currently used for OA and the risk of joint replacement surgery (10) and for the reduction of the economic and social burden caused by this disease, finding new therapeutic options for OA has always been considered.
There is no proven intervention to restore cartilage. Therefore, recent pharmacological investigations have been focused on joint structure-modifying capacities and slowing the mechanism of the disease (22). Disease-modifying osteoarthritis drugs have been proposed as desirable adjuncts to symptomatic relief and joint structure reconstruction; however, the efficacy of these modulators is still uncertain in numerous studies and requires further research (18).
Evidence exists that calcitonin inhibits collagenase and phospholipase A2 activity in isolated human OA articular chondrocytes while affecting cartilage development (17). By applying isolated chondrocytes from fetal calf epiphysis, an experimental study reported that calcitonin could induce the engagement of glucosamine into glycosaminoglycans (23). In another experimental double-blind study on 30 guinea pigs, the results of intraperitoneal (IP) and subcutaneous (SQ) calcitonin injections (30 IU/kg) for 7 days were compared to the control group. They measured the development of periosteum, cortical, and trabecular bones in all groups, with significant differences in periosteal formation between the study groups (P = 0.009). It is worth noting that those who received IP had higher levels of periosteal formation than the SQ injection group. There was no significant difference in the formation of cortical and trabecular bone between the study groups in the second and fourth weeks. According to their findings, systemic calcitonin (in high doses) could effectively improve periosteum formation at the early stage of bone healing (24), which is in line with findings on the positive effects of calcitonin’s chondroprotectiveness.
Another study, aiming to assess the impact of nasal form calcitonin on knee OA and QoL in women, was conducted on 220 postmenopausal women with knee pain and knee OA. The Western Ontario and McMaster Universities Osteoarthritis Index, the QoL questionnaire of the European Foundation for Osteoporosis (QALEFFO-41), and the visual analog scale were applied to evaluate algofunction. The participants reported considerable declines in pain and stiffness and improvement in functional ability and total scores of WOMAC and QUALEFFO, which were significant following 90 days of treatment. Researchers concluded that providing nasal calcitonin was associated with the stimulation of dual action on osteoporosis and OA with considerable enhancements in QoL and algofunction (25). In line with the aforementioned findings, the current study’s results also revealed that calcitonin elicits declines in pain, stiffness, and movement in OA that were significant during the first month using WOMAC and NRS indicators.
In a prospective cross-sectional study, which aimed to assess the impacts of weekly administration of calcitonin on 28 female cases using the WOMAC questionnaire, significant improvements were observed in pain, joint stiffness, functional ability, and total WOMAC score following 5 weeks of treatment. It was concluded that calcitonin provides increased locomotor activity, improvement of QoL, and appropriate rehabilitation, which are major parameters for those who suffer from OA (26), which is in line with the results of the present study.
In summary, the evidence provided by numerous experimental studies (24, 27) indicated dual actions of calcitonin on bone and cartilage as its direct chondroprotective effects. Calcitonin not only prevents osteoclastic bone resorption but also maintains the structural uniformity of subchondral bone components (28). As indicated by the present study and other studies, calcitonin causes appropriate functional outcomes and decreased pain severity as the ultimate outcome of all interventions. Such evidence provides clues about the benefits of calcitonin for OA management and as a DMOAD in future therapeutic guidelines.
5.1. Conclusions
The present study’s findings revealed that the weekly administration of 50 IU calcitonin for 4 weeks could significantly improve physical ability and pain intensity in patients with knee OA. However, further multi-central studies efforts with a larger sample size are required to further approve the findings and enable the expansion of new horizons in OA treatment.