1. Background
Ensuring adequate brain perfusion in the prevention of cerebral ischemia/hyperemia is significant among anesthesiologists. Cerebrovascular autoregulation causes the relatively stable regulation of cerebral blood flow (CBF) against changes in blood pressure (1). Therefore, maintaining blood pressure in an area that supports the autoregulatory function of the brain is crucial for anesthesiologists. Oxidative stress surprisingly causes systemic ischemia/reperfusion injury that leads to brain damage, endothelial dysfunction with cardiovascular failure, and death (2, 3). These consequences can be a strong rationale for targeting oxidative stress with antioxidant therapy. Vitamin C has a protective effect against ischemia/reperfusion injury (4).
Numerous pieces of evidence have shown the effect of oxidative status on controlling vascular tone (5). The evidence has indicated that the oral intake of vitamins C and E causes the prevention of flow-mediated dilation decrease noticed following an oral glucose load or prolonged sitting (6). Additionally, the intravenous injection of ascorbic acid has been shown to have a direct vasodilation impact on the inoculated vessel, and oral intake of a fruit and vegetable purée-based drink, recognized to remarkably raise plasma vitamin C, increases endothelium-dependent vasodilation (7). Lately, vitamin C has also been shown to affect cerebral vascular regulation in an opposite manner; the acute increase in CBF or perfusion, induced by a single self-contained underwater breathing apparatus dive with exposure to hyperoxia, was decreased in individuals who received vitamin C supplementation for 6 days (8-10). In this regard, a fascinating finding was the significant reduction of functional magnetic resonance imaging signal changes during standard tasks through vitamin C intravenous administration (11). The aforementioned observations bring into question the possible effects of vitamin C on brain oxygenation during anesthesia.
Cerebral oximetry measures tissue oxygenation by measuring the transcutaneous frontal cortex (12). This method is currently widely used in neonatal, pediatric, thoracic, vascular, cardiac, neurological, and neuropathic medicine (13). The mentioned method is non-invasive monitoring of cerebral blood oxygen saturation with an emphasis on venous blood. Studies have shown that variables, such as CBF, the cerebral metabolic rate of oxygen, and mean arterial pressure (MAP), can affect this technique (14-16).
2. Objectives
The current study was designed and performed to assess the effect of vitamin C infusion and brain oxygenation with cerebral oximetry on improving brain perfusion during general anesthesia in vascular surgery of diabetic patients.
3. Methods
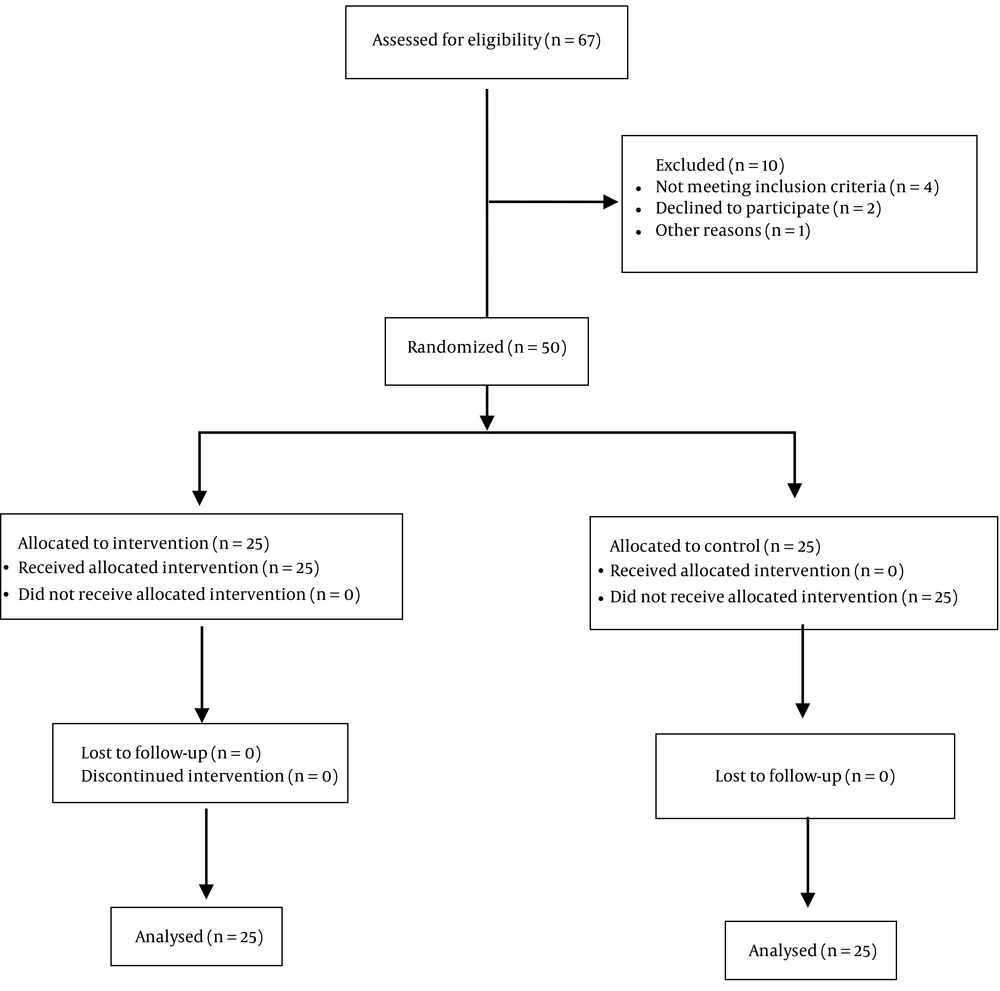
This double-blind clinical trial (registration no.: IRCT20210830052345N1) was approved by the Ethics Committee of Biomedical Research of Shahid Beheshti University of Medical Sciences, Tehran, Iran (code: IR.SBMU.MSP.REC.1398.167). Patients who were candidates for vascular surgery under general anesthesia and referred to Taleghani Hospital in Tehran during 2019 - 2020 were included if they met the inclusion criteria (candidates diagnosed as the American Society of Anesthesiologists (ASA)-II diabetic patients and aged > 50 years). The exclusion criteria were III ≤ ASA, hemoglobin < 8 g/dL, brain failure, metabolic disease, and any type of intracranial pathology. and after providing full explanations and obtaining written consent. The research physicians were blinded to the patient groups. Moreover, the patients were double-blind to the injected drugs. A total of 50 patients were included in the study. Afterward, the patients were randomly divided into two groups, vitamin C (intervention) and placebo (Figure 1).
The patients were randomly and non-blindly divided into groups, including the placebo and vitamin C groups, using the randomized block method in the form of four blocks. In the operating room, after connecting non-invasive blood pressure monitoring, the clinical symptoms of all patients, including pulse oximetry, blood sugar (BS), cerebral oximetry, blood pressure, and heart rate (HR), were recorded. Then, in the placebo group, the patients received an infusion of 500 mL of isotonic saline half an hour before anesthesia induction. In the vitamin C group, half an hour before anesthesia induction, 1 g of vitamin C diluted in 500 mL isotonic saline was given to the patients as a mixed infusion of vitamin C.
Cerebral oximetry was placed on both sides of the patient’s forehead, and oxygenation was continuously monitored. The patients were placed in a supine position for 10 minutes. Before anesthesia induction, the HR, BS, MAP, and brain tissue oxygen saturation percentage (e.g., regional oxygen saturation (rSO2), peripheral oxygen saturation (SpO2), partial pressure of carbon dioxide (PaCO2), supercritical carbon dioxide (sCO2), and end-tidal carbon dioxide (ETCO2)) were recorded. In addition, the aforementioned parameters were recorded after anesthesia induction and at the end of the surgery. Brain SpO2 was continuously monitored bilaterally, which was recorded before and after anesthesia and at the end of the surgery.
The patients were given 0.07 g/kg of midazolam and 2 μg/kg of fentanyl intravenously. Anesthesia was induced by the intravenous injection of 5 mg/kg sodium thiopental and 0.5 mg/kg atracurium besylate. To maintain anesthesia, isoflurane gas, and air/O2 combination at a ratio of 50% were used. Intravenous atracurium 0.2 mg/kg and fentanyl 1 μg/kg were considered for necessary cases. At the end of the surgery, neostigmine 40 μg/kg and atropine 15 μg/kg were used to reverse the effects of atracurium. Pulse oximetry was also used to determine oxygenation. This study reported a more than 15% increase in sCO2 relative to baseline cerebral hyperperfusion, and a 13% decrease in sCO2 relative to baseline was called cerebral ischemia. The bispectral index of all patients was maintained within the range of 40 - 60 during anesthesia. The data obtained from the patients were analyzed using SPSS software (version 23; IBM Endicott, New York, the USA). Descriptive reports, including frequency tables and graphs of qualitative variables and central indices and dispersion of quantitative variables, were obtained. The correlation coefficient was used to check the relationship between the variables. A t-test was used to compare values between the two groups. Significance was considered at the 5% level.
4. Results
In this study, a total of 67 patients were included within 2019 - 2020, 50 subjects of whom, with an average age of 69.24 ± 9.46 years, had the necessary data for the final evaluation. According to the results in Table 1, the ratio of male to female patients was almost 1.13 times.
| Variables | Placebo Group | Vitamin C Group | Total Groups |
|---|---|---|---|
| Age (y) | 71.56 ± 8.89 | 66.92 ± 9.61 | 69.24 ± 9.46 |
| Weight (kg) | 83.68 ± 10.51 | 86.72 ± 13.82 | 85.2 ± 12.25 |
| Height (cm) | 174.20 ± 9.97 | 169.52 ± 9.03 | 171.86 ± 9.71 |
| Gender | |||
| Male | 14 (56) | 13 (52) | 27 (54) |
| Female | 11 (44) | 12 (48) | 23 (46) |
| Total | 25 (100) | 25 (100) | 50 (100) |
Demographic Information of Patients a
According to the Geisser-Greenhouse test (Table 2), no significant difference was observed in systolic and diastolic blood pressure, SpO2, MAP, HR, PaCo2, rSO2, sCO2, and ETCO2 in the total groups and between the two groups in the three times before and after anesthesia induction and at the end of surgery. Furthermore, there was no significant difference in BS levels between the groups; however, the BS levels in total groups decreased significantly (P < 0.05). Therefore, there was a significant difference between the BS levels before and after anesthesia induction and at the end of surgery in total groups.
| Variables | Type III Sum of Squares | df | Mean | F | P-Value |
|---|---|---|---|---|---|
| SBP total | 21.00 | 1.70 | 12.29 | 0.23 | 0.75 |
| SBP between two groups | 0.33 | 1.70 | 0.19 | 0.04 | 0.99 |
| DBP total | 9.33 | 1.07 | 8.68 | 0.10 | 0.76 |
| DBP between two groups | 36 | 1.07 | 33.51 | 0.39 | 0.54 |
| HR total | 43.61 | 1.17 | 37.28 | 1.58 | 0.21 |
| HR between two groups | 0.25 | 1.17 | 0.21 | 0.09 | 0.94 |
| BS total | 1264.36 | 1.04 | 1209.8 | 36.21 | 0.01 |
| BS between two groups | 0.65 | 1.04 | 0.62 | 0.01 | 0.90 |
| MAP total | 31.00 | 1.80 | 17.17 | 2.22 | 0.12 |
| MAP between two groups | 282.33 | 1.80 | 156.42 | 20.22 | 0.40 |
| PaCo2 total | 4.48 | 1.32 | 3.39 | 1.37 | 0.25 |
| PaCo2 between two groups | 2.08 | 1.32 | 1.57 | 0.64 | 0.46 |
| SpO2 total | 6.09 | 1.03 | 5.91 | 0.80 | 0.37 |
| SpO2 between two groups | 4.57 | 1.03 | 4.43 | 0.60 | 0.44 |
| rSO2 total | 1.29 | 1.05 | 1.22 | 0.07 | 0.79 |
| rSO2 between two groups | 1.21 | 1.05 | 1.15 | 0.07 | 0.79 |
| sCO2 total | 920.09 | 1.47 | 624.86 | 20.4 | 0.06 |
| sCO2 between two groups | 4.17 | 1.47 | 2.83 | 0.09 | 0.85 |
| ETCO2 total | 2.41 | 1.23 | 1.95 | 0.76 | 0.41 |
| ETCO2 between two groups | 1.24 | 1.23 | 1.00 | 0.39 | 0.57 |
| BIS |
Comparison of Measured Parameters in Groups
As can be observed in Table 3, the amount of cerebral perfusion of the two groups under study and the three evaluated stages, including before and after anesthesia and at the end of the surgery, does not show a difference (P > 0.05). In addition, the investigated variables for perfusion and cerebral ischemia were stable in the three mentioned stages and had no difference between the two investigated groups (P > 0.05).
| Group | Hyperperfusion Before Anesthesia | Hyperperfusion After Anesthesia | Hyperperfusion at the End of Surgery | Cerebral Ischemia Before Anesthesia | Cerebral Ischemia After Anesthesia | Cerebral Ischemia at the End of Surgery | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | |
| Vitamin C | 100 | - | 100 | - | 100 | - | 100 | - | 100 | - | 100 | - |
| Placebo | 100 | - | 100 | - | 100 | - | 100 | - | 100 | - | 100 | - |
Crosstab of Hyperperfusion and Cerebral Ischemia
5. Discussion
Diabetes mellitus is one of the most important risk factors for stroke, and it seems that diabetic patients can benefit from carotid endarterectomy. Patients undergoing carotid endarterectomy will reach optimal conditions from a clinical point of view if they suffer very few complications during the operation. Therefore, it is crucial to pay attention to the patient’s clinical conditions and consider the possibility of any kind of risk after the operation. In many cases, stroke and heart attack have been reported as the cause of patient mortality.
It is crucial to check and control the side effects caused by the methods used for anesthesia. In the last decade, numerous studies have been conducted to choose the most appropriate anesthesia method in endarterectomy surgery. These studies examined cerebral complications, mortalities, and myocardial infarction (17, 18).
Since patients who are candidates for endarterectomy often suffer from ischemic heart disease, one of the most important challenges for these patients is hemodynamic instability (19). In patients undergoing anesthesia and endarterectomy surgery, blood pressure drop might be observed due to factors, such as the suppression of the sympathetic nervous system or reduction of cardiac preload (20). Additionally, cerebral hyperperfusion syndrome after carotid endarterectomy is common in patients with carotid stenosis or chronic hypertension (5-7). The increase of nitric oxide during the occlusion of the internal carotid artery and the release of oxygen-derived free radicals during brain perfusion repair can be effective in disrupting endothelial function and self-regulation mechanisms after endarterectomy (21). One of the important antioxidants in brain metabolism is vitamin C, which has been reported to have neuroprotective effects on reperfusion syndrome (20).
The results of the current study showed that using vitamin C did not significantly change the oxygen index of patients and improve their clinical condition, compared to the placebo group. The mechanism of antioxidants’ synergistic effect on intravenous anesthetics is not precise. The aforementioned antioxidant effects are identical to the effects of antioxidants in the body, such as vitamin C. Egwu et al. showed that vitamin C caused an extended sleep time in rabbits following xylazine administration, which might be resulted from vitamin C’s membrane stabilizing impact, thereby hindering the further movement of xylazine into and out of the brain (22). As a result, the present study hypothesized that the probable mechanism of the synergistic effect of vitamin C is the impact of pretreatment with vitamin C on membrane stability. However, it is required to prove the mechanism of the synergistic impact of antioxidants on intravenous anesthetics.
The obtained results of the present study, in comparison to other studies, can be due to the integration of two strategies to treat carotid stenosis, namely drug therapy, and surgery. Furthermore, using drugs during surgery is one of the strengths and innovations of this project. However, there are a few points, such as the limited number of samples (especially in the intervention group) and not using different doses of vitamin C. Using higher doses of vitamin C can cause different conditions. However, the use of animal models and other various indicators can also provide more accurate information.
5.1. Conclusions
The analysis of the findings showed that the effect of vitamin C infusion and evaluation of brain oxygenation with cerebral oximetry on improving brain perfusion during general anesthesia is similar to the placebo in vascular surgery. However, what is important is the stability of patients’ hemodynamic parameters. Therefore, it can be said that using vitamins in diabetic patients undergoing carotid endarterectomy can maintain the hemodynamic status of patients in stable conditions.