1. Background
Postoperative pain is one of the most important problems after surgery that causes distress for the patient, prolongs hospital stay, and increases cost. Using analgesics alone, such as narcotics or non-steroidal anti-inflammatory drugs, cannot effectively control moderate to severe pain (1). Femoral bone fractures in middle or old age cause severe pain after surgery and might lead to complications, such as delayed movement, deep vein thromboembolism, and bed sores. Various methods, such as spinal and epidural, are used to control postoperative pain (2).
Epidural anesthesia is one of the most effective methods for lower extremity surgery that relieves not only pain during orthopedic surgery but also postoperative pain. Epidural anesthesia can provide quick mobilization, enhanced recovery of function, and a decrease in cardiopulmonary morbidity in the initial postoperative period (3). The epidural method reduces the physiological stress associated with surgery and causes better surgical outcomes and decreased pain after surgery (4). The local anesthetics administration in actual doses can cause side effects, such as hypotension, bradycardia, and motor weakness. Therefore, several adjuvants, such as opioids (5), clonidine (6), ketamine (7), and dexamethasone (8), have been presented for epidural practice.
Various studies have been performed to investigate multidrug treatment for intraoperative and postoperative pain because no single drug has yet been found to have the necessary efficacy without extensive side effects (9). The use of adjuvants in postoperative analgesia has become commonly used, which leads to better quality and prolongation of the postoperative block. The use of ropivacaine, which is a long-acting amide, is widely used to induce adequate anesthesia after orthopedic surgery (10-12); nevertheless, due to its potential side effects, such as motor block and central nervous system toxicity, the use of the lowest possible dose is recommended (13).
Currently, fentanyl is widely used for anesthesia and pain relief. This drug is one of the short-acting synthetic drugs. Drugs, such as fentanyl, are usually used as adjuvants in combination with local anesthetics to increase the effect on the epidural block (14, 15). Nevertheless, fentanyl, similar to other opioids, increases the chances of urinary retention, respiratory depression, and nausea and vomiting (16, 17).
One of the selective α2 agonists is dexmedetomidine, which has a beneficial effect when used in the epidural method (18). By affecting the pre- and post-nerve endings, dexmedetomidine reduces the release of norepinephrine from sympathetic terminals, thereby causing sedation and analgesic properties, and hemodynamic effects during surgery (19, 20). This drug has been used for epidural anesthesia in several surgeries (6, 15, 21-23). In some studies, morphine has been used as an agent in epidural anesthesia (24, 25); however, due to its longer respiratory depression effects.
2. Objectives
This study aimed to use fentanyl. The present study compared dexmedetomidine to fentanyl as an adjunct to ropivacaine for epidural anesthesia in patients undergoing femoral neck fracture surgery.
3. Methods
3.1. Trial Design
This study was performed as a double-blind clinical trial after obtaining permission from the Ethics Committee of Ahvaz Jundishapur University of Medical Sciences (IR.AJUMS.REC.1398.987) in Imam Khomeini hospital, Ahvaz, Iran.
3.2. Ethical Statement
The ethics code of this study was established by the Pain Research Center, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran (IR.AJUMS.REC.1398.987) (IRCT no.: IRCT20200613047760N1 26/08/2020). The objectives and potential risks and benefits of the study were explained to the patients, and informed consent was obtained from all patients.
3.3. Randomization and Blindness
People were divided into two groups randomly based on the random block permutation method. For example, for blocks of four, we imagined 6 blocks ABBA, AABB, ABAB, BABA, BBAA, and BAAB, which should be n/4. These blocks were sampled by placement. A random follow-up was obtained from https://www.sealedenvelope.com. The individuals who were blind in the study process included the patients, the surgeon, and the investigators. The surgical procedure was performed by the same surgeon. A computer-generated allocation-concealment process was used before recruiting. The injectable drugs were equipped by the investigator and entitled 1 and 2.
3.4. Sample Size
The difference between the postoperative analgesia of dexmedetomidine and fentanyl was considered at about 27 minutes. Power of 80% and α = 0.05 produced a sample size of 26 patients per group. However, the sample size was improved to 28 patients in each study group to consider the 10% drop in patient numbers or missing data points.
3.5. Participants
The study was conducted on 56 patients who underwent elective femoral fracture surgery by epidural anesthesia in Imam Khomeini hospital, Ahwaz, Iran, within September 2019 to May 2020.
The inclusion criteria were: (1) American Society of Anesthesiologists class І and ІІ; (2) the age range of 20 - 70 years; and (3) candidate for elective femoral fracture surgery.
Exclusion criteria were: (1) cardiovascular disease; (2) coagulation disorders; (3) spinal deformity; (4) epidural site infection; (5) obesity (body mass index (BMI) > 30 kg/m2); (6) amide-type local anesthetics or dexmedetomidine; (7) block failure; and (8) surgery duration longer than 3 hours.
3.6. Study Settings and Intervention
In the operating room, after visiting and initial examinations, all patients were taken intravenously with a size 18 intravenous catheter. The patients were hydrated with 10 mL/kg isotonic solution during 15 - 20 minutes. Regular monitoring, including pulse oximetry, non-invasive blood pressure, and electrocardiography, was carried out. Baseline vital signs were recorded. Epidural anesthesia was performed for all patients in the sitting position after disinfecting the injection site in the space between the upper lumbar spine (L4 - L5) with a disposable 18-gauge Tuohy needle (B. BRAUN company). Epidural anesthesia was performed using the hanging drop technique from the midline position. After the injection of the test dose (with 5 mL of lidocaine 2% with adrenaline 1/200000), a single-hole catheter is gently inserted into the epidural space, pushed into the epidural space about 3.5 - 4 cm, and fixed there.
All patients were injected with 150 mg of ropivacaine 7.5% solution (AstraZeneca Company, France). The participants were randomly allocated into two groups, group D (1 μg/kg of dexmedetomidine (Jinan Haili Biotechnology Co., Ltd., China)) and group F (1 μg/kg of fentanyl (Aburaihan Co., Iran)). The total volume of loading dosage injected solution was 20 mL (diluted in stilled water) in all patients. Whenever the visual analog scale (VAS) score was higher than 3, subsequent doses, including 75 mg of ropivacaine as the rescue dose, were prescribed through the catheter. A nasal mask with an oxygen flow of 6 liter/minute was installed for all patients after epidural anesthesia.
3.7. Outcomes
Primary outcomes included:
(1) The onset and duration of sensory block; this was checked every 2 minutes by the cold sponge till it extended the level of T10. The duration of the sensory block was monitored at 15-minute intervals until it reached the level of L53;
(2) The onset and duration of a motor block were based on the Bromage scale (26). The onset and duration of motor block were monitored every 5 minutes in the first 30 minutes and then every 30 minutes until the Bromage scale reached 0;
(3) The analgesia duration (based on the VAS score) (27).
The operation started once the motor block extended to grade 3 on the Bromage scale.
The secondary outcome was the sedation score. It was evaluated by five points sedation scale at intervals of 15 minutes during surgery and intervals of 60 minutes after the end of surgery (28).
Heart rate, blood pressure, and peripheral oxygen saturation (SPO2) as the hemodynamic parameters were recorded immediately after receiving the studied drugs (examination times in the first 15 minutes, every 5 minutes, then every 15 minutes until the end of the operation, and then in the 1st, 2nd, 4th, 6th, 12th, and 24th hours after the operation.
The benchmark for the assessment of analgesia is the VAS score. Pain is graded from 0 to 10 according to the VAS criteria. If during the operation to 24 hours postoperatively, the VAS score was higher than 3, 75 mg of ropivacaine was injected through an epidural catheter. The amount of prescribed ropivacaine was recorded in the next 24 hours. The VAS score is a VAS that, based on the intensity of pain, is given a score from 0 to 10, with 0 and 10 indicating no pain and the worst amount of pain imaginable, respectively (27).
Complications, such as itching, urinary retention, nausea and vomiting, respiratory depression, chills, and hypotension, were recorded and treated. The definition of hypotension was a reduction of extra than 20% mean arterial pressure relative to the starting point or lower systolic blood pressure below 90 mmHg treated with crystalloid infusion or 6 mg ephedrine blues injection. Bradycardia, a heart rate below 50 beats per minute, was treated with a 0.5 mg intravenous atropine injection.
3.8. Statistical Analysis
Statistical summaries are reported in the form of tables and graphs. Quantitative variables and qualitative variables are reported as numbers. Statistical analysis was carried out using SPSS software (version 22). Moreover, the relationship between variables and demographic information was evaluated using the chi-square test, Mann-Whitney U test, and t-test. The significance level of the tests was 0.05. The value of P was considered. The repeated measures analysis of variance was used for the heart rate and mean arterial pressure data analysis. Two P-values were reported for time and grouping factors. Bonferroni’s post-hoc test was used for intergroup analysis.
4. Results
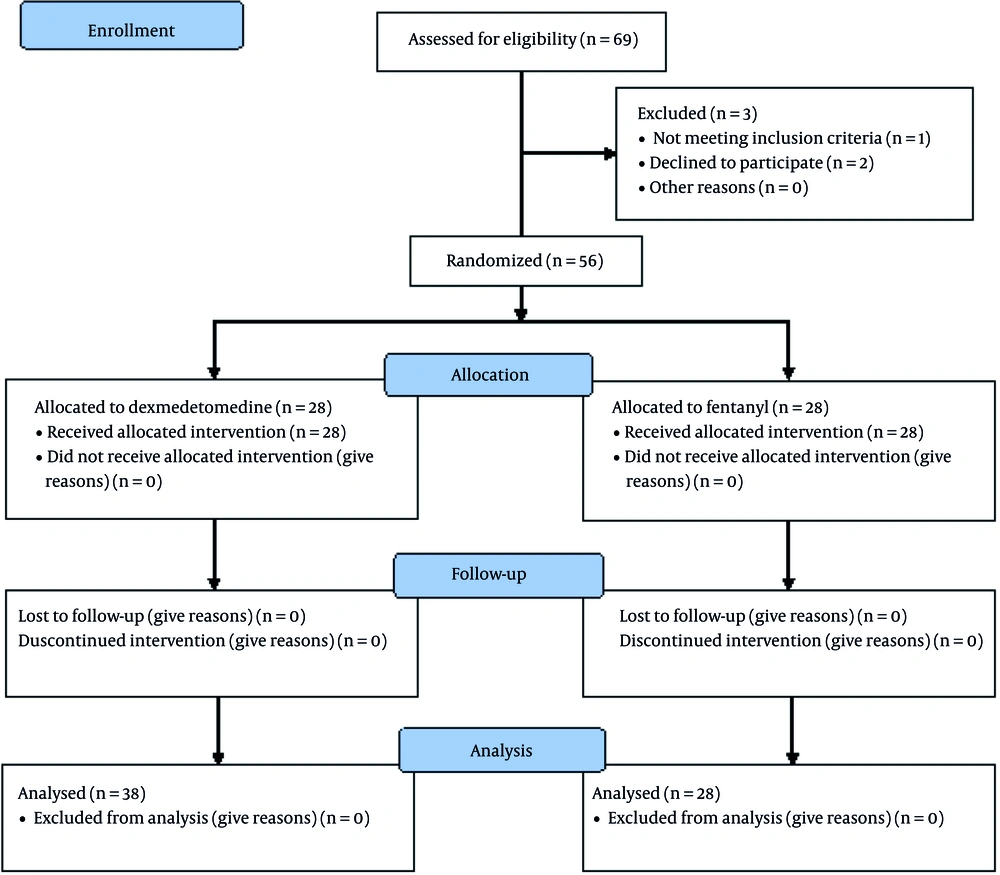
Throughout the study period, within September 2019 to May 2020, 80 participants under elective femoral fracture surgery were eligible to contribute to the trial. Among the 80 participants, 69 patients consented to participate in the trial, and only 56 patients were enrolled and randomized. One patient did not meet the entry criteria, and two patients failed in epidural anesthesia. The patients were allocated into dexmedetomidine (n = 28) and fentanyl (n = 28) groups (Figure 1).
The demographic and surgical information were comparable in terms of mean age, gender, BMI, and duration of surgery between the groups, which were not significantly different in both groups (P > 0.05; Table 1). The duration of the block in the dexmedetomidine group was higher than in the fentanyl group (P = 0.045); therefore, the onset time of the sensory and motor block was lower in the dexmedetomidine group than in the fentanyl group (P < 0.001). The VAS score in the dexmedetomidine group was lower than in the fentanyl group, and the highest VAS score was reported in the fentanyl group (5.8 ± 0.9 vs. 4.9 ± 0.6; P < 0.001).
| Variables | Group F (n = 28) | Group D (n = 28) | P-Value |
|---|---|---|---|
| Age (y) | 53.6 ± 19.3 | 47.7 ± 15.5 | 0.221 |
| Gender (male/female) | 15 - 13 | 12 - 16 | 0.442 |
| Body mass index (kg/m2) | 25.6 ± 0.6 | 24.5 ± 0.5 | 0.129 |
| Surgery duration (min) | 101.35 ± 10.54 | 104.32 ± 8.64 | 0.213 |
The patients of the dexmedetomidine group required 2.54 ± 1.36 μg rescue doses during surgery and in the next 24 hours. However, in the fentanyl group, the need to prescribe the drug was 3.15 ± 1.64 μg rescue doses. This difference was statistically significant (P < 0.05; Table 2).
| Variables | Group F (n = 28) | Group D (n = 28) | P-Value c |
|---|---|---|---|
| Block duration (min) | 226.6 ± 46.1 | 311.2 ± 60.3 | 0.045 d |
| Onset time of sensory block (min) | 6.0 ± 1.1 | 3.5 ± 0.6 | < 0.001 d |
| Onset time of motor block (min) | 22.6 ± 2.2 | 17.5 ± 1.9 | < 0.001 d |
| Highest visual analog scale score | 5.8 ± 0.9 | 4.9 ± 0.6 | < 0.001 d |
| Rescue doses during and 24 hours after surgery (mg) | 3.15 ± 1.64 | 2.54 ± 1.36 | < 0.05 d |
Regarding the sedation score, the initial sedation score was similar in the two groups (P > 0.05). Fifteenth minutes after epidural anesthesia, the sedation score of the fentanyl group was higher than the dexmedetomidine group (1.07 vs. 0.03; P < 0.05). The sedation score was higher from the 30th minute (P = 0.01) to the 120th minute (P = 0.04) in the patients of the dexmedetomidine group than in the fentanyl group. Hemodynamic parameters and SPO2 were similar in the two groups (P > 0.05). The average intraoperative mean arterial blood pressure was different between the groups (P < 0.05; Table 3).
| Hemodynamic Variables | Group F (n = 28) | Group D (n = 28) | P-Value | P-Value Bonferroni’s Test |
|---|---|---|---|---|
| Heart rate | < 0.0001 c, 0.3425 d | |||
| Before epidural anesthesia | 106.7 ± 16.4 | 104.5 ± 13.5 | > 0.9999 | |
| After epidural anesthesia | 105.7 ± 14.4 | 102 ± 13.6 | 0.5826 | |
| During surgery | 112.7 ± 18.6 | 107.3 ± 14.2 | > 0.9999 | |
| Mean arterial pressure | < 0.0001 c, 0.0975 d | |||
| Before epidural anesthesia | 101.4 ± 21.4 | 93.0 ± 15.2 | 0.2926 | |
| After epidural anesthesia | 79.8 ± 11.4 | 87.1 ± 10.4 | 0.0012 e | |
| During surgery | 94.4.4 ± 16.7 | 97.5 ± 16.3 | 0.0457 |
Dry mouth, hypotension, and bradycardia were more common in the dexmedetomidine group. Moreover, nausea and vomiting, chills, and itching were more common in the fentanyl group. There was no respiratory depression in both groups. There was no significant difference between the two groups regarding the aforementioned complications (P > 0 .05). The need for the administration of ephedrine in the intraoperative period was more in the fentanyl group (8 ± 2 mg) than in the dexmedetomidine group (6 ± 3 mg).
5. Discussion
The present study compared the quality of analgesia of 1 μ/kg of dexmedetomidine to 1 μ/kg of fentanyl in combination with 150 mg of ropivacaine in the epidural method for patients undergoing orthopedic femoral neck fracture surgery. There was no difference in age, gender, BMI, and surgery duration between the two groups. The duration of sensory block onset at the T10 level was shorter in the dexmedetomidine group than in the fentanyl group. In the dexmedetomidine group, the block length was longer than in the fentanyl group (311.2 ± 60.3 vs. 226.6 ± 46.1 minutes; P < 0.045).
Postoperative pain management creates a major problem for practitioners caring for patients. Moreover, good control of postoperative pain is significant in avoiding many complications, such as pulmonary, metabolic, and psychological (29). Numerous types of research have recommended the use of postoperative epidural analgesia in high-risk patients for decreased complications (30-32).
This study’s hypothesis was that dexmedetomidine was a better epidural adjuvant to ropivacaine when compared to fentanyl for obtaining early onset and prolonged postoperative epidural analgesia. The results of the present study confirmed the aforementioned hypothesis.
The effect of dexmedetomidine compared to morphine as an adjuvant with bupivacaine in orthopedics fracture surgery was studied by Gousheh et al. (33). The block duration was longer in the dexmedetomidine group than in the morphine group (266.9 ± 5.9 vs. 237.8 ± 4.0 minutes; P < 0.001), which confirms the results of the current study (33).
The duration of onset of sensory block up to the T10 level in the fentanyl group was longer than in the dexmedetomidine group (6.0 ± 1.1 vs. 3.5 ± 0.6 minutes). In a study by Kaur et al. to evaluate the effect of ropivacaine in comparison to ropivacaine and dexmedetomidine on epidural anesthesia in patients with lower limb fractures, the onset of sensory block was shorter in the dexmedetomidine group (12.536 ± 4.172 vs. 14.182 ± 6.020 minutes; P = 0.115), which is consistent with the results of the current study (34). Giri et al. showed that adding dexmedetomidine to ropivacaine caused a shorter onset of sensory analgesia at T10 (8.52 ± 2.36 minutes) than to ropivacaine alone (9.72 ± 3.44 minutes) (35). Additionally, they determined that analgesia duration (342 minutes; P < 0.05) and time of motor block (246.72 ± 30.46 minutes) was longer in the group of dexmedetomidine (35). The results of the aforementioned study are consistent with the results of the present study in this regard.
The onset of motor block in the dexmedetomidine group was shorter than in the fentanyl group (17.5 ± 1.9 vs. 22.6 ± 2.2 minutes; P < 0.001). These results are similar to Akhondzadeh et al.’s results (36). They examined the effect of dexmedetomidine with lidocaine in the supraclavicular block for forearm fracture surgery. They showed that the onset of sensory and motor block in the dexmedetomidine group was shorter. Furthermore, the duration of sensory and motor block was longer, and the request for analgesia was more than control groups (36).
Regarding the highest VAS score and rescue doses during and up to 24 hours after surgery, the highest VAS score in the dexmedetomidine group was lower than in the fentanyl group (4.9 ± 0.6 vs. 5.8 ± 0.9). In addition, rescue doses during and 24 hours after surgery were lower in the dexmedetomidine group than in the fentanyl group (2.54 ± 1.36 vs. 3.15 ± 1.64 mg). A study conducted by Ayyappan and Santhanakarishnan compared the efficacy of epidural bupivacaine with dexmedetomidine to epidural bupivacaine with fentanyl for postoperative pain relief (37). They concluded that dexmedetomidine is a better adjuvant than fentanyl in epidural bupivacaine. It can cause faster sensory and motor block, longer postoperative analgesia, and lower consumption of rescue analgesia. The aforementioned results are consistent with the results of the present study (37).
The results showed that patients in the dexmedetomidine group had higher and more visible sedation scores than patients in the fentanyl group. Oriol-López and Maldonado-Sánchez, as cited in Chiruvella et al., conducted a prospective study on 40 patients undergoing abdominal surgery with epidural anesthesia (6). They compared 1 μg/kg of dexmedetomidine to 3 mg/kg of lidocaine and epinephrine. The sedation score was obtained, according to Ramsey, as cited in Chiruvella et al. Ramsey’s score was 3 at 5 minutes and 3 - 4 within 15 - 90 minutes. They showed that acceptable sedation was obtained within 10 and 120 minutes with a single bolus epidural dose of dexmedetomidine (6). The aforementioned result is consistent with the results of the present study, Bajwa et al.’s study (38), and Akhondzadeh et al.’s study (36).
5.1. Study Limitations
This study had some limitations. The first one was determining the exact doses of dexmedetomidine and fentanyl, which was not investigated in this study and should be addressed in future studies. The second one was the small sample size in this study, which was conducted in one center. It is suggested to perform this study in the future with more participants and multiple centers. The third limitation was conducting this study in a non-trauma center. For this reason, there was a wide age range for the patients. It is suggested to follow this method in other centers with younger patients.
5.2. Conclusions
Overall, this study concluded that dexmedetomidine as an adjuvant in epidural anesthesia for orthopedic femoral fracture surgery shortens the onset time of sensory and motor block, increases the duration of analgesia, and prolongs the duration of anesthesia. Sedation with dexmedetomidine is more suitable than fentanyl and has fewer side effects.