1. Background
In cardiac surgery, anesthetics significantly affect the course of the intraoperative period and the success of the postoperative period. Total intravenous anesthesia (TIVA) and inhalation anesthesia are traditional methods of anesthesia in cardiac surgery.
Cardiac surgical procedures unavoidably cause myocardial cell injury originating from myocardial ischemia/reperfusion, cardiopulmonary bypass (CPB), operative procedures, etc. (1-5).
The medical community is increasingly aware of the need for quality patient care. Anesthesiologists, in particular, are demonstrating leadership in quality and safety. Cardiothoracic anesthesiologists can improve the quality of care provided to cardiac patients, both through specific anesthetic techniques and in a team approach with other specialists during surgery (6). Numerous studies have demonstrated the superiority of intraoperative administration of halogenated agents over propofol in myocardial revascularization surgery (7-10).
Cardiac surgery in adults is associated with the occurrence of postoperative complications (11). Even minor complications can increase the cost of treatment. Given the potentially preventable nature of these postoperative complications, preventive methods should be used to improve outcomes after cardiac surgery. One of them is the choice of anesthetic technique (12). Nevertheless, most of those studies were conducted on patients undergoing coronary artery bypass graft (CABG) surgery. Furthermore, high-quality meta-analyses in adult cases have shown controversial results (7, 13-17).
2. Objectives
The effect of inhalation and TIVA has been extensively studied on CABG surgery, but the effect of these anesthetics on hemodynamics, oxygen transport, and energy consumption on mitral and aortic valve replacement has not been studied at all. Accordingly, this study was conducted to determine the effects of sevoflurane, isoflurane, and propofol on the cardiac index (CI) and metabolic outcomes during aortic and mitral valve replacement in adults.
3. Methods
3.1. Study Design
This single-center prospective randomized controlled clinical study was approved by the Ethics Committee of Astana Medical University (code: 3). Written informed consent was obtained from all subjects. This manuscript followed the Consolidated Standards of Reporting Trials (CONSORT) guidelines (Figure 1) and was registered on the ClinicalTrials.gov website (code: NCT05696509).
The examination and treatment data of 75 patients operated in the Cardiosurgical Department of the Medical Center Hospital of the Presidential Administration of the Republic of Kazakhstan were included in the study. All patients underwent mitral and aortic valve replacement/plasty under CPB conditions. This research was conducted between 2020 and 2022. To calculate the sample size, we used the following formula:
allowing us to identify the static significance of the study.
3.1.1. Inclusion Criteria for the Main Study Phase
(1) The age between 40 - 60 years old;
(2) mitral valve insufficiency grade 3 - 4;
(3) aortic valve insufficiency grade 3 - 4;
(4) participants of both sexes;
(5) planned surgical interventions;
(6) signed informed consent.
3.1.2. Exclusion Criteria
(1) Pregnancy;
(2) hypertensive disease;
(3) coronary artery disease;
(4) current unstable angina pectoris;
(5) preoperative hemodynamic instability, defined as the use of vasopressors.
Only elective patients with the American Society of Anesthesiologists (ASA) physical status II - III were included in this study.
A total of 75 patients were randomly assigned into 3 groups according to the type of anesthesia: The propofol group (n = 25), the sevoflurane group (n = 25), and the isoflurane group (n = 25).
The study was conducted in 5 stages:
(1) Patient’s baseline value determination before anesthesia;
(2) after tracheal intubation;
(3) before the CPB;
(4) after the CPB;
(5) postoperative period until the patient is extubated.
Before induction of anesthesia, hemodynamic monitoring was started on admission to the operating theatre using a Nihon Kohden monitor (Japan). The right radial artery was catheterized for invasive monitoring of systemic arterial pressure and arterial blood sampling. A catheter was then inserted into the central jugular vein and guided into the right atrium under ultrasound guidance for mixed venous blood sampling.
Cardiac stroke volume (SV) was determined by intraesophageal echocardiography.
Then, cardiac output (CO):
and CI:
were determined. Next, the blood oxygen content was determined using the following formula:
where CaO2 is arterial ABG and CvO2 is central mixed venous ABG.
After that, oxygen delivery was determined using the formula:
Oxygen consumption during surgery:
Or
Also, we used this formula to find:
In the second stage, after tracheal intubation, indirect calorimetry was used to determine VO2 and energy expenditure during anesthesia using a spirometry device (Oxford, UK), which was connected to an endotracheal tube and continuously showed oxygen demand and energy expenditure. A transesophageal echocardiography sensor was used to determine CO. Additionally, CO was determined by the Fick principle. The same tests (CO, CI, consumption, oxygen delivery, and energy expenditure) were performed in the third and fourth stages of the study. In the last stage, the consumption of muscle relaxants and opioid analgesics was calculated to assess the pharmacy efficiency of anesthetics. The time of extubation and the time of patient transfer to the specialized department were also calculated.
All patients were anesthetized with fentanyl (5 - 7 μg/kg), ketamine (1.5 - 2 mg/kg), and propofol (1 - 1.5 mg/kg) intravenously fractionally. Pipecuronium bromide (0.04 - 0.07 mg/kg) was used as a muscle relaxant in all patients. To maintain anesthesia in the propofol group, propofol was used intravenously as an anesthetic at a dose of 4 - 6 mg/kg/h using a perfusor (BBRAUN). In the sevoflurane group, sevoflurane was used as an anesthetic at a dose of 1.7 - 1.9 MAC. In the isoflurane group, isoflurane was used as an anesthetic at a dose of 1.1 - 1.2 MAC. To determine the pharmacological efficacy of anesthetics, fentanyl was fractionally administered intravenously at a dose of 100 μg when the heart rate and blood pressure increased. Pipecuronium bromide at a dose of 2 mg was used intravenously for muscle relaxation.
Inhalational anesthetics were used from the beginning of surgery to CPB and after CPB. It was used until the end of surgery.
During CPB, propofol at a dose of 6 mg/kg/h was used intravenously via perfusion in all patients. Besides myorelaxant pipecuronium bromide at a dose of 2 mg every 40 - 60 minutes, fentanyl was used intravenously at a dose of 100 μg every 30 minutes for anesthesia. Norepinephrine solution was administered intravenously at a dose of 0.05 μg/kg/min via perfusion and dobutamine at a dose of 5 μg/kg/min after CPB in all patients at the same dosages.
The purposes of cardiotonic preparations:
(1) To maintain mean arterial perfusion pressure (CPB causes cytokine storm and vasodilation);
(2) for inotropic support (for reperfusion syndrome, resulting in a lower ejection fraction).
The depth of anesthesia was monitored with a processed electroencephalogram in the form of a bispectral index.
Statistical analysis was performed using SPSS version 20 (SPSS Inc, Chicago, IL, USA) using 1-factor analysis of variance (ANOVA) for independent samples and nonparametric Kruskal-Wallis test. The Kruskal-Wallis test was only used to estimate myorelaxant consumption, as this parameter produced an abnormal distribution. Pearson and Spearman correlation analyses were also performed to determine the significance of the association between CI and oxygen consumption and energy expenditure.
4. Results
All groups of patients were comparable in terms of anthropometric data, age, weight, baseline hemodynamic status, and blood oxygen transport function (Table 1).
| Indicator | Propofol (n = 25) | Sevoflurane (n = 25) | Isoflurane (n = 25) |
|---|---|---|---|
| Sex | |||
| Male | 19 | 22 | 21 |
| Female | 6 | 3 | 4 |
| Age (y) | 58.1 ± 9.1 | 56.4 ± 10.5 | 59.2 ± 8.2 |
| Weight (kg) | 81.4 ± 9.6 | 80.5 ± 6.6 | 78.2 ± 6.2 |
| Height (cm) | 165.1 ± 4.5 | 170 ± 6.3 | 165.5 ± 6.7 |
| Duration of surgery (h) | 4.2 ± 0.7 | 4.0 ± 0.7 | 3.9 ± 0.8 |
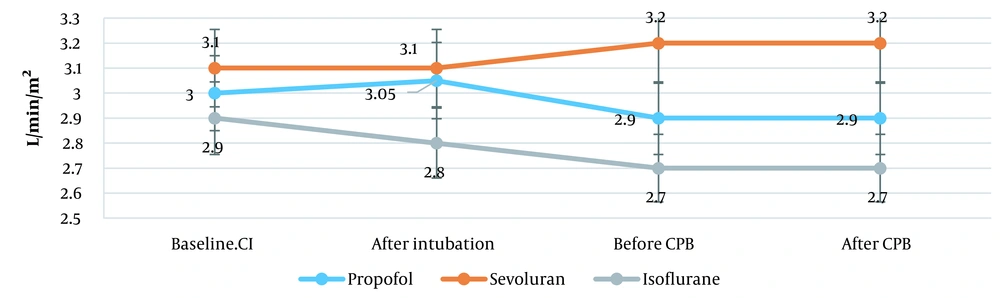
| CI (L/min/m2) | 3.0 ± 0.8 | 3.1 ± 0.7 | 2.9 ± 0.5 |
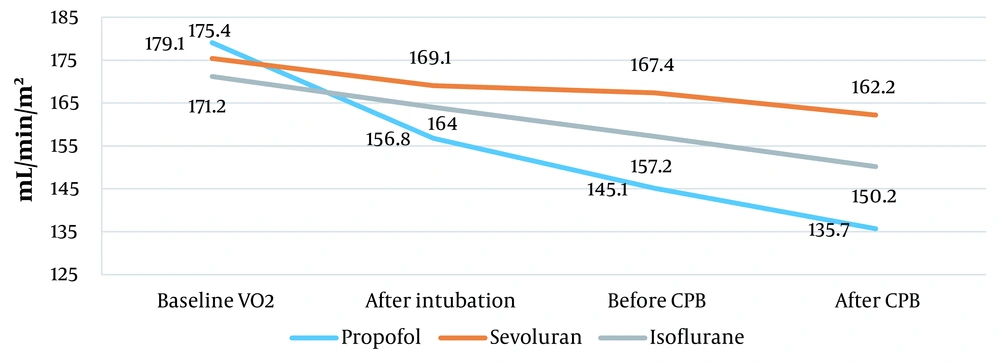
| Oxygen consumption (mL/min/m2) | 171.5 ± 29.8 | 173.4 ± 28.7 | 174.2 ± 25.3 |
| TPR (dyne/s/cm-5) | 3186 ± 697.6 | 2891.1 ± 634 | 3084 ± 635.6 |
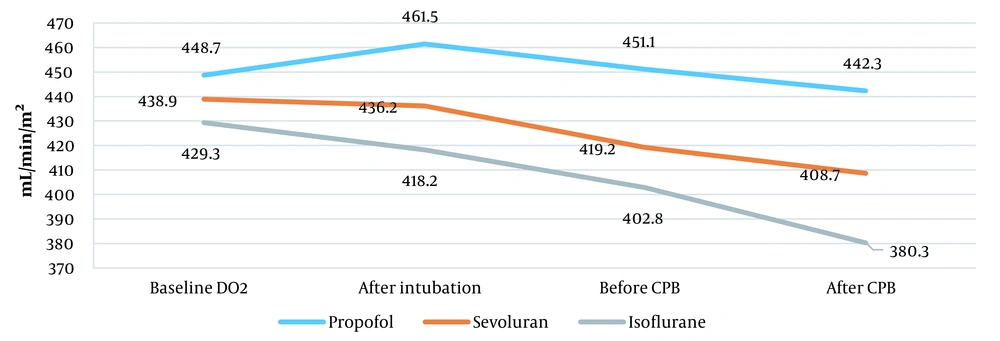
| Oxygen delivery (mL/min/m2) | 454.7 ± 72.5 | 438.9 ± 82.8 | 439.3 ± 66.5 |
Demographic, Anthropometric Data, Surgical Volume, Cardiac Index, Oxygen Consumption, Oxygen Delivery, and Total Peripheral Vascular Resistance a
During heart valve replacement, in the propofol group, CI slightly decreased from 3.0 ± 0.8 to 2.9 ± 0.8 L/min/m2 (3.3%). In the sevoflurane group, CI slightly increased from 3.1 ± 0.7 to 3.2 ± 0.7 L/min/m2 (3.2%) before entering CPB. After that, its rise was not observed; it remained at this level until the end of surgery. In the isoflurane group, CI slightly decreased from 2.9 ± 0.5 to 2.7 ± 0.6 L/min/m2 before entering CPB. Then, there was no change in CI in the isoflurane group (P = 0.001; Figure 2).
A significant reduction in oxygen consumption was observed in the propofol group, from 179.1 ± 29.8 to 145.1 ± 30.9 mL/min/m2 before CPB and to 135.7 ± 16.9 mL/min/m2 after CPB. Isoflurane was the most effective inhalational anesthetic agent, reducing oxygen consumption from 171.2 ± 25.3 to 157.2 ± 35.3 mL/min/m2 before CBP and to150.2 ± 38.3 mL/min/m2 after CBP. The effect of sevoflurane on oxygen consumption was not significant. It reduced oxygen consumption from 175.4 ± 28.7 to 167.4 ± 39.8 mL/min/m2 before CBP and to 162.2 ± 52 mL/min/m2 after CBP (P = 0.02; Figure 3). Total peripheral vascular resistance (TPR) was almost at the same level before anesthesia.
However, propofol significantly decreased TPR from 3186 ± 697.6 to 1993.5 ± 404.2 dyne/s/cm-5. Isoflurane decreased TPR from 3084 ± 635.6 to 2475.8 ± 343.0 dyne/s/cm-5. However, during anesthesia with sevoflurane, TPR did not decrease significantly; it decreased from 2891.1 ± 634 to 2756.4 ± 484.2 dyne/s/cm-5.
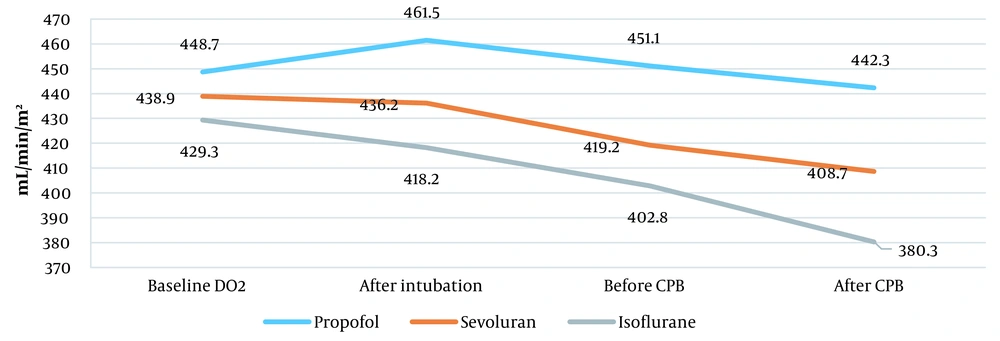
Oxygen transport increased from 448.7 ± 72.5 to 461.5 ± 71.5 mL/min/m2 after tracheal intubation in the propofol group. However, it decreased to 451.1 ± 89.4 mL/min/m2 before entering СРВ and to 442.3 ± 58.5 mL/min/m2 after the end of surgery. Inhalational anesthetic sevoflurane decreased oxygen transport from 438.9 ± 82.3 to 408.7 ± 35.3 mL/min/m2. Also, isoflurane significantly reduced oxygen transport from 439.3 ± 66.5 to 380.3 ± 47.1 mL/min/m2 (P = 0.02; Figure 4).
Energy expenditure decreased from 1483.7 ± 195.1 to 1333.5 ± 69.2 kcal in the propofol group. However, in the sevoflurane group, energy expenditure increased from 1550.2 ± 117.3 to 1572.2 ± 66.5 kcal. In addition, energy consumption decreased in the isoflurane group from 1315 ± 140.1 to 1245.3 ± 71.9 kcal before entering CPB. After CPB, its increase was observed up to 1258.8 ± 74.7 kcal (P = 0.0001; Figure 5).
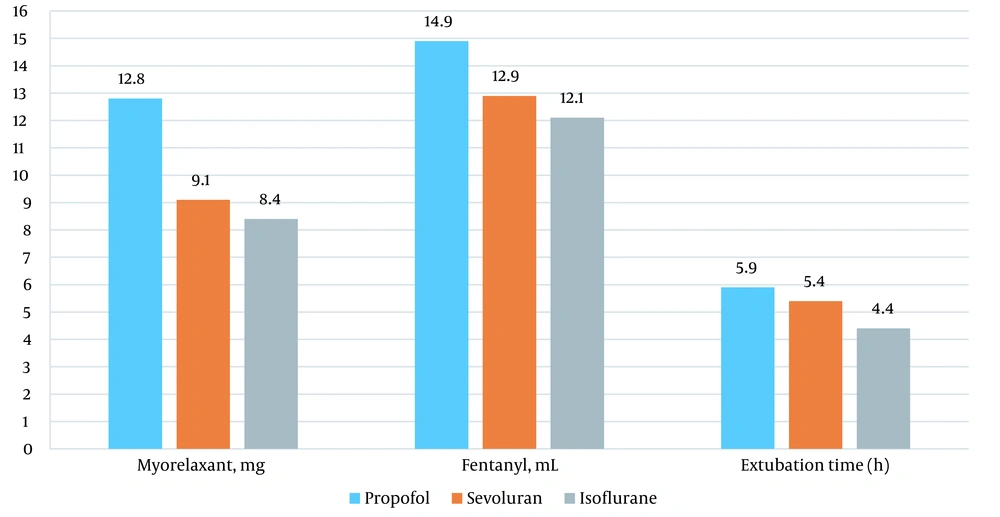
More muscle relaxants and fentanyl were used in the propofol group than in the inhaled anesthetic group. In addition, after anesthesia with propofol, patients were on longer mechanical ventilation in the postoperative period (Figure 6).
Correlation analysis was performed using the Pearson method, as the distribution was normal in the Smirnov-Kolmogorov test. The correlation between CI and oxygen consumption in heart valve replacement was insignificant, and the strength of the relationship was very weak, as here, P = 0.16 equaled R = 0.1. In addition, the correlation between CI and energy expenditure was very weak and insignificant, as here, P = 0.15 equaled R = 0.1.
5. Discussion
The halogen-containing preparations (i. e., sevoflurane, isoflurane, TIVA with propofol) are commonly used for anesthesia.
In this study, age, weight, risk of surgery, and intraoperative and postoperative complications were almost the same in all patients in the 3 groups. This study aimed to assess the effect of isoflurane, sevoflurane, and TIVA with propofol on the main hemodynamic indicator (CI) and the metabolic response of the body during cardiac surgery for aortic and mitral valve replacement.
For a number of years, there has been a search for preparations for anesthesia, providing both anesthesia and protection of the myocardium from anoxic damage during cardiac surgery. Isoflurane and sevoflurane have shown cardioprotective effects (18).
A systematic review and meta-analysis found some evidence of cardioprotective effects of volatile anesthetics in CABG with an increase in CI and a decrease in the use of inotropic preparations. However, the dose and timing of inhalation anesthetics for myocardial protection should be further studied. With the use of isoflurane and sevoflurane, we observed only minor changes in CI, which were within the normal range. We found that inhalation anesthetics sevoflurane at a dose of 1.7 - 1.9, and isoflurane at a dose of 1.1 - 1.2 did not cause changes in CO; they are optimal for anesthesia of such patients. However, it is reasonable to prescribe these volatile anesthetics with great caution in patients with preoperative changes in hemodynamics and heart rate because they can cause myocardial depression, vasodilation, or prolongation of the QT interval on the electrocardiogram (ECG) (19). Sevoflurane decreased TPR from 2891.1 ± 634 to 2756.4 ± 484.2 dyne/s/cm-5, and isoflurane decreased TPR from 3084 ± 635.6 to 2475.8 ± 343.0 dyne/s/cm-5. These results confirm the need for further studies on the study of hemodynamics during anesthesia in cardiac surgery with these anesthetics.
Propofol is a general anesthetic widely used during heart surgery. In addition to its anesthetic effect, propofol has been reported to provide myocardial protection during cardiac surgery and reperfusion (20). To explain this cardioprotective effect, various mechanisms have been proposed, including inhibition of calcium channels and blockade of free radicals (21, 22). However, some studies have compared sevoflurane and propofol during CABG, showing that only sevoflurane has a protective effect on the myocardium (7, 23). According to the researchers, the use of propofol can adversely affect myocardial function. However, the discrepancy between the data on the cardioprotective effect on the myocardium (20) and its negative effect on it (7, 23) can be explained by the dosing regimen of the preparation (24). In our study, the use of propofol at a dose of 4 - 6 mg/kg/h did not change CI; it increased by only 3.2%. We believe that the use of propofol at such a dose is optimal for anesthesia during aortic and mitral valve replacement in these patients. The mechanisms that control the oxygen distribution in the body are not fully understood (25). It was revealed that oxygen consumption decreased after inhalation anesthesia and increased during surgery. Jakobsson et al. (26) noted its decrease after induction of anesthesia by an average of 34% and 2 hours after surgery by 24%. Changes in VO2 were paralleled by disturbances in oxygen delivery and utilization. General anesthesia reduced oxygen consumption, oxygen delivery, and oxygen extraction in elderly patients. These changes in these indicators affect the oxygen transport function of the blood and require further assessment (27). Oxygen delivery is an important marker of oxygen transport, and its range of 330-500 mL/min-1 is a good indicator during anesthesia (28). Bacher et al. (29) determined the delivery of oxygen and calculated its content in arterial blood. CO2 and energy consumption were determined using indirect calorimetry. Hypothermia in patients during anesthesia has been shown to decrease the metabolic rate but does not change DO2. According to Hausmann et al. (30), oxygen consumption during general anesthesia did not depend on the type of anesthetic administered. Abad Gurumeta and Lopez Quesada (31) showed that during prolonged anesthesia, an increase in the need for O2 could have an adverse effect on hemodynamics. The researchers believe that immediate action should be taken to reduce VO2 when O2 intake increases above the limit during anesthesia. This can lead to complications (31). Our study revealed that VO2 increased with a decrease in its delivery during inhalation anesthesia with sevoflurane and isoflurane, and vice versa; when using TIVA with propofol, oxygen consumption did not increase, and its delivery to tissues increased.
Indirect calorimetry allows identifying energy costs during surgery. Often used in practice, sevoflurane increases energy costs, while propofol reduces energy expenditure during anesthesia.
The choice of optimal anesthesia methods for cardiac surgeries is of great importance. At the same time, the use of certain preparations for TIVA anesthesia with propofol or inhalation anesthetics is often explained by personal experience of their use, force of habit, and traditions of this department.
Based on the literature, the effects of sevoflurane, isoflurane, and TIVA with propofol on hemodynamics in the form of changes in CI, oxygen transport blood function (VO2 and DO2), and energy consumption during cardiac surgery are heterogeneous.
Our study showed that in anthropometrically homogeneous patients with low surgical risk, sevoflurane, isoflurane and TIVA with propofol did not cause significant hemodynamic changes. However, inhalation anesthetics reduce TPR, which can lead to hypotension. Impairment of the circulatory system and intraoperative complications can occur due to the wrong dose of the preparation.
Total intravenous anesthesia with propofol is accompanied by a decrease in oxygen consumption and an increase in its delivery to tissues with lower energy costs (detected by indirect calorimetry) compared to inhalation anesthetics. These data should be considered in high-risk patients for surgery and anesthesia.
5.1. Limitations
This study has 2 limitations. The first limitation is that this study is a single-center study. The second limitation is the small sample size because the sample size affects the statistical significance of the study. Further randomized controlled studies with larger sample sizes are needed.
5.2. Conclusions
Sevoflurane, isoflurane, and TIVA with propofol had no intraoperative complications or effect on CI in patients with a low risk of ASA surgery. Anesthesia with propofol was accompanied by a lower VO2 and better oxygen delivery to tissues. Energy expenditure in TIVA with propofol was reduced.