Dear Editor,
Presently, 7 known coronavirus strains lead to human infection, 4 of which (ie, 229E, NL63, HKU1, and OC43) induce cold symptoms in immunocompromised individuals (1). Studies have indicated that SARS-CoV-2, a new species of the genus Betacoronavirus, is responsible for the recent SARS outbreak, demonstrating the most virulent epidemic impact among Coronaviridae with neurological manifestations (2).
Neurological symptoms are common in COVID-19 patients. In a study of 17806 patients with SARS-CoV-2 admitted to 2 university hospitals in Adana City between March 11, 2020, and January 1, 2021, Boz et al. observed that patients with higher inflammatory markers and older patients were more likely to experience seizures during SARS-CoV-2 infection (3).
Regarding SARS-CoV-2 neuroinvasion, there are several hypotheses based on previous evidence on coronaviruses with similar characteristics, but the precise signaling pathways of SARS-CoV-2 have yet to be elucidated (4). This study investigated the possible mechanisms associated with COVID-19 infection that lead to seizures.
A- Direct Pathway
Seizures are a well-known consequence of meningitis and encephalitis. Moriguchi et al. reported a case of SARS-CoV-2–induced meningoencephalitis that predominantly prompted generalized seizures (5). Emami et al. (6) concluded that new seizures in critically ill patients with COVID-19 should be considered symptomatic acute seizures; also, Zeng et al. (7) indicated that of the 1237 patients with epilepsy, 31 patients (8.33%) experienced increased seizures during the pandemic.
Reportedly, SARS-CoV-2 causes viral meningoencephalitis and acute necrotizing encephalitis (ANE) (8). To uncover these results, we must investigate the direct entry of the virus into the brain via transsynaptic and hematologic pathways. Transsynaptic spread is one of the possible direct pathways of SARS-CoV-2 neuroinvasion. A study found that up to 80% of COVID-19 patients had significant olfactory dysfunction (9). In addition, studies have indicated that the virus may infect the brain through neural invasion and retrograde transsynaptic spread after being inoculated into the cornea, retina, lung, and gut (10).
Hematogenous spread is an additional method of brain invasion for which there are 2 hypotheses:
1- Express Angiotensin-Converting Enzyme 2
The capillary endothelium of the central nervous system’s (CNS) microcirculation expresses angiotensin-converting enzyme 2 (ACE-2), the receptor for SARS-CoV-2. This results in endothelial damage and the virus’s entry into the brain (11).
In the study by Russell et al., it was evidenced that the virus attacked the blood-brain barrier (BBB) by expressing ACE-2 and related viral entry receptors in dendritic cells and macrophages after attacking the vascular endothelium (12). Seizures may result from the overproduction of angiotensin II, causing severe acute lung injury, vasoconstriction, and oxidative processes that promote brain degeneration (13).
2- Trojan Horse Mechanism
This mechanism represents leukocyte migration into brain tissue. In other words, the virus utilizes these cells as a reservoir to infect brain tissue. As a result, the systemic inflammation associated with COVID-19 increases the permeability of the BBB, rendering it easier for infected immune cells to invade the CNS (14, 15).
B- Cytokine Storm
Multiple studies have shown a correlation between seizures, inflammation, and elevated cytokine levels. Interleukin 1 beta (IL-1β), IL-6, and tumor necrosis factor α (TNF-α) have been extensively studied regarding their convulsive effects.
Concerning COVID-19, cytokine release syndrome (CRS) is characterized by high levels of inflammatory cytokines, such as IL-6 and TNF-α (16). According to Guirao et al., IL-6 plays an important role in disease progression and can thus be a useful biomarker for determining disease severity. To this end, tocilizumab is recommended as an effective treatment option for IL-6 enhancer disorder (17).
Nikbakht et al. concluded that the neurological symptoms of COVID-19 infection in the brain are caused primarily by the entry of pro-inflammatory cytokines into the nervous system or through the production of these cytokines by microglia and astrocytes. Pro-inflammatory cytokines can disrupt the BBB, increase glutamate and aspartate levels, decrease γ-aminobutyric acid (GABA) levels, and interfere with ion channel function. Finally, high levels of cytokines can induce epilepsy (18) (Figure 1).
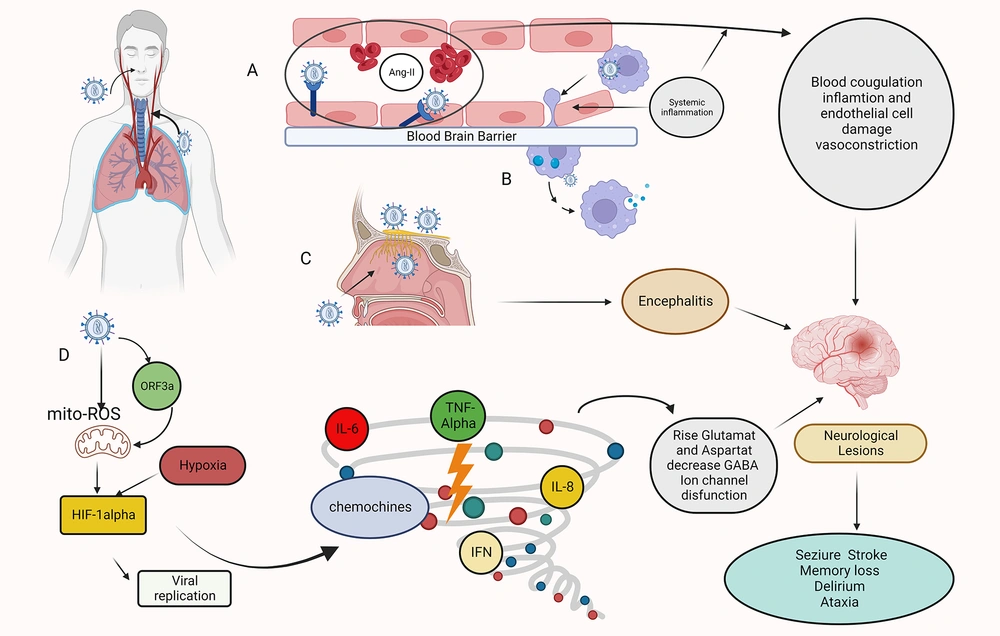
Mechanisms of Sars-COV-2 invasion of the CNS. Hematogenous route: A. A transcellular migration occurs when the virus binds to its receptors, ACE2, or neuropilin-1 (NRP-1), on brain microvasculature endothelial cells and crosses the endothelial cell via transcytosis. SARS-COV-2 depletes the ACE2 receptor by infecting target cells, including endothelial cells, resulting in the accumulation of angiotensin II (ANGII). A high level of ANGII can cause vasoconstriction, fluid retention, inflammation, and blood coagulation, resulting in an ischemic or hemorrhagic stroke. B. Infection immune cells, which then carry the virus across the blood-brain barrier (BBB) endothelial cells into the CNS (Trojan Horse mechanism). Transsynaptic route: C. The SARS-COV-2 infects the olfactory epithelium ,reaches the CNS through the olfactory neurons, and causes encephalitis. Other mechanisms: D. Role of HIF-1α in hypoxia signaling in covid-19. Infection with SARS-COV-2 causes severe inflammation, acute respiratory distress syndrome (ARDS), and hypoxia in the lungs. As a result, hypoxia and inflammation induced encephalopathy and seizure occur. When Sars-cov-2 entering host cells, viral ORF3a protein induces HIF-1α expression through triggering mitochondrial reactive oxygen species (ROS) activation. The accumulated HIF-1α leads to a cytokine storm. This figure was created by Seyed Amir Abbas Ahadia with Biorender.com.
C- Hypoxia and Other Mechanisms
Identifying the effective factors in reducing COVID-19 patients’ respiratory function could save many lives.
Hypoxia can exacerbate hypoxic encephalopathy, which can contribute to seizure development. Ischemic brain damage also contributes to hypoperfusion and may lead to seizures (13).
Zalpoor et al. indicated that COVID-19 might play a role in epilepsy and seizures through hypoxia-inducible factor 1 alpha (HIF-1α) stimulation and P2X7 receptor hyperactivation, where P2X7 receptor blockers may be a promising strategy to prevent or consider treating neurological complications in COVID-19 patients with epilepsy (19).
Hypoxemia induced by SARS-CoV-2 may also be caused by acute acquired porphyria introduced by a viral invasion of hemoglobin’s 1-beta chain. By attacking hemoglobin and destroying heme, ORF3a protein (a conserved coronavirus protein involved in virus replication and release) damages respiratory tissue. Consequently, patients develop respiratory distress, coagulation symptoms, and metabolic disorders (20, 21).
Moreover, other mechanisms, such as astrocyte swelling and GABA ERGIC deficiency at synapses, may also explain post-hypoxic seizures (11).
According to Habas et al., electrolyte abnormalities are not uncommon among SARS-CoV-2 patients, and hyponatremia is considered one of these electrolyte disorders. The syndrome of inappropriate antidiuretic hormone secretion (SIADH) is the most prevalent cause of hyponatremia and can initially manifest as seizures (22, 23).
Finally, a significant increase in blood clots in patients with COVID-19 may be attributed to hypercoagulable states and a high rate of cerebrovascular accident (CVA) in patients with thrombotic microangiopathy (TMA). If seizures accompany SARS-CoV-2 infection after a CVA, acute symptomatic seizures should be considered (23-26).
Conclusions
Since most studies focus primarily on respiratory symptoms, the prevalence of neurological complications associated with COVID-19 may be underreported.
Seizures are a common neurologic complication in COVID-19 patients. Nevertheless, the mechanism by which COVID-19 patients develop seizures remains unknown. To reduce patient morbidity and mortality, clinicians should strive to identify inciting factors (hypoxia, fever, sepsis, and electrolyte derangements) as soon as possible and apply general seizure management principles when treating COVID-19 patients who experience seizures. To this end, a thorough investigation of COVID-19’s neurological symptoms, manifestations, complications, and underlying pathophysiological mechanisms is warranted. Eventually, the medical society must not ignore the possibility of neurological harm among people with mild to moderate infection.