1. Background
For more than a century, the brachial plexus block has been an effective technique for anesthesiologists to create regional anesthesia that is comparable to general anesthesia (1). Due to the creation of anesthesia and analgesia during surgery, as well as the relief of postoperative pain, the progress in upper limb surgeries is largely due to the brachial plexus block technique. In addition, with the advent of ultrasound (US)-guided techniques, the role of brachial plexus block in upper limb surgeries has become increasingly prominent (2). Brachial plexus block in upper limb surgeries is performed with different techniques (neurostimulation, US, or transarterial) depending on where the brachial plexus is available (interscalene, supraclavicular, infraclavicular, axillary, and humeral canal) (3). Because the 3 main nerves of this plexus, which are the main nerves of the upper extremity, are surrounded by a sheath of connective tissue in the axilla, filling this sheath with a single injection of local anesthetic (LA) can provide safe, rapid, and effective anesthesia in the upper extremities during forearm and hand surgeries (4). On the other hand, blocking the brachial plexus in the axilla area has no side effects (such as pneumothorax, phrenic nerve injury, and recurrent laryngeal nerve injury), occurring in other methods of blocking this plexus (5). Therefore, the axillary block of the brachial plexus can be one of the most suitable block methods for this plexus. By performing axillary block of the brachial plexus, the inner part of the arm is not completely anesthetized because this part of the arm is innervated by the lateral cutaneous branch of the second intercostal nerve (intercostobrachial nerve (ICBN)) and the medial branch of the brachial cutaneous nerve (6). The intercostobrachial nerve is not a branch of the brachial plexus; thus, it is out of reach of LA during the axillary block. Pressure on this nerve by closing the tourniquet to control bleeding in the surgical field after 30 to 45 minutes can lead to relatively severe pain that can interfere with the upper limb surgery process (7). According to these explanations, ICBN block (in addition to axillary block) can be a logical solution to control the pain caused by closing the tourniquet in the upper limb. Blocking this nerve can be done by 2 methods: (1) LA injection in the nerve pathway using superficial nerve anatomy (along the axillary vein in the midaxillary line); and (2) LA injection with US guidance and thus selective ICBN blockade (8). Since ICBN is a pure sensory nerve and a neurostimulator cannot be used for its block, conventional methods and landmarks have been used in some diagnostic and therapeutic cases. Today, with the introduction of US in the field of nerve blocks, the accuracy of nerve identification and block performance has increased, and the complications of performing a blind block (such as perforation of blood vessels, hematoma, and the entry of LA into the circulatory system) have decreased. Therefore, in this research, besides examining the effect of ICBN block on tourniquet pain, a comparison was made between the 2 block methods under US guidance and without it (conventional method).
2. Objectives
Since no study has been performed to compare the effectiveness of these 2 methods in ICBN block and tourniquet pain management in the axillary block of brachial plexus, the present study aimed to compare ICBN block with and without US guidance in controlling pain caused by a tourniquet during the axillary block in soft tissue surgery of the hand and forearm.
3. Methods
3.1. Study Design and Patients
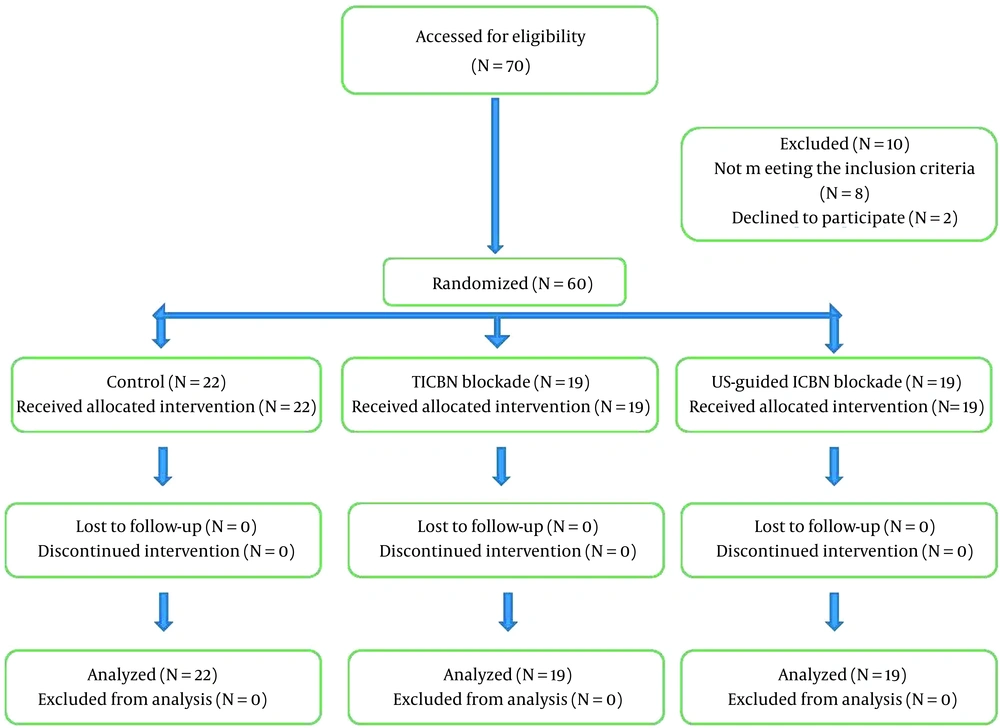
This randomized, double-blind, controlled clinical trial was performed on 60 patients who were candidates for elective soft tissue surgery of the hand and forearm referred to Hazrat-e Fatemeh Hospital, Iran University of Medical Sciences (IUMS), Tehran, Iran, from 2018 to 2019 according to the Consolidated Standards of Reporting Trials (CONSORT) statement (Figure 1). The design and implementation of this clinical trial were registered in the research system of the Vice Chancellor for Research and Technology of IUMS. Then, all stages of the project were performed after obtaining the ethics code from the Ethics Committee of IUMS (code: IR.IUMS.FMD.REC.1398.046). This study was also registered on the Iranian Registry of Clinical Trials website (code: IRCT20170301032837N3). The research team adhered to the ethical principles of the Helsinki Convention on clinical trials at all stages of the study. All patients entered the study after receiving full explanations about the study and providing written consent.
3.2. Sample Size
The sample size was calculated based on similar studies and G*Power software. Considering a 20% loss, the final volume was calculated by 20 people in each group and a total of 60 people.
3.3. Eligibility Criteria
Inclusion criteria were age between 20 and 50 years, insensitivity to LA drugs, ASA physical status I - II, indication of forearm or hand soft tissue surgery lasting at least 90 minutes, and body mass index between 23 and 28. Exclusion criteria were drug addiction, coagulation problems, upper extremity neuropathy, vasculitis, unstable hemodynamics, and a history of seizures or mental illness.
3.4. Grouping
After applying the inclusion and exclusion criteria, the patients were randomly divided into 3 groups: the control group (n = 22), the traditional ICBN (TICBN) blockade group (n = 19), and the US-guided ICBN blockade group (n = 19).
Randomization was performed as permuted block randomization (4 in each block) without stratification on baseline characteristics by a computer program. Randomization and assignment of patients to study groups, collection of patients’ data after the intervention, and analysis and interpretation of data were each performed by separate researchers, each of whom was blinded to the study groups. Before the intervention, all demographic characteristics were collected and recorded using predetermined forms.
3.5. Intervention
Before performing the axillary block of the brachial plexus in patients who were candidates for surgery, an intravenous catheter (G20) was inserted into the patient’s vein to inject crystalloids and sedatives (a vein was taken from the patient). Midazolam (0.05 mg/kg) and fentanyl (1.5 μg/kg) were injected preoperatively to induce sedation. After vital signs monitoring (non-invasive blood pressure, pulse oximetry, heart rate, and electrocardiogram), the intervention was performed as follows. The axillary block was performed by injecting 30 mL of 1.5% lidocaine using US guidance and a neurostimulator. Next to each nerve, 6-8 mL of 1.5% lidocaine was injected.
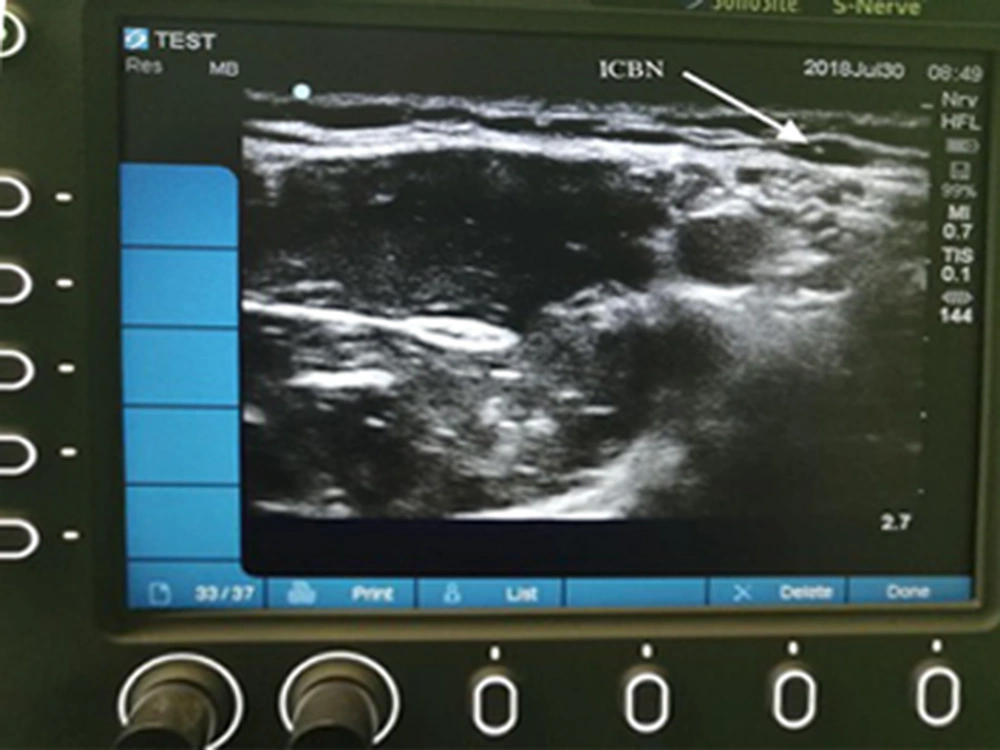
In the control group, only the axillary block of the brachial plexus was performed without ICBN block. In the TICBN blockade group, in addition to the axillary block of the brachial plexus, the ICBN block was performed without US guidance and based on the traditional method using superficial anatomy and nerve pathway by subcutaneous injection of 2 mL of 1.5% lidocaine at the site of the axillary pulse. In the US-guided ICBN blockade group, in addition to the axillary block of the brachial plexus, the ICBN block was performed under US guidance. No neurostimulator was used because the ICBN is a sensory nerve. Thus, after the axillary block and before the needle was removed, the location of the ICBN was identified by sonography on the axillary artery and superficial to deep fascia; the block was performed by injecting 2 mL of 1.5% lidocaine (Figure 2).
3.6. Outcome Assessment
The duration of pain onset was recorded in minutes from the moment of inflating the tourniquet on the arm until the expression of tourniquet pain. Pain intensity for all patients was scored between 0 (painless) and 10 (most painful) based on the Numeric Rating Scale (NRS).
3.7. Data Analysis
After collecting data, all data were analyzed using SPSS version 22 (SPSS Inc, Chicago, IL, USA). Mean and SD were used to report quantitative variables. Frequency (%) was used to report qualitative variables. The normality of the distribution of variables was determined using the Kolmogorov-Smirnov test. Due to the independence of the study groups and assuming that the distribution of variables was normal, the t test was used to compare variables within each of the 2 groups. Analysis of variance (ANOVA) was used to compare variables between the 3 groups. If the distribution of variables was not normal, the nonparametric Mann-Whitney test was used to compare the variables within each of the 2 groups. The nonparametric Kruskal-Wallis test was used to compare variables between the 3 groups. The chi-square test was used for the statistical analysis of qualitative variables between the 3 groups. P values less than 0.05 were considered statistically significant.
4. Results
4.1. Demographic Characteristics
The mean age of patients was 30 ± 8.82 (age range, 18 to 56 years). All patients participating in the study were male. The overall mean blood pressure was 124.41 ± 17.27 mmHg (in the range of minimum 14 and maximum 151 mmHg). The overall mean heart rate was 75.88 ± 8.12 bpm (range, 60 to 100 bpm). The overall mean for the duration of surgery was 119.53 ± 35.11 minutes (range, 50 to 250 minutes). The mean body mass index was 23.23 ± 1.87 kg/m2. Eighty percent of patients had ASA physical status I, and 20% had ASA physical status II. Wrist surgery was the most common (51%) type of surgery in the patients. The overall mean tourniquet pressure was 227 ± 16.18 mmHg. The tourniquet’s average active and inactive time, respectively, was 13.13 ± 2.7 and 14.62 ± 2.51 minutes. The results showed no significant differences between the 3 groups in demographic and clinical variables at the beginning of the study (P > 0.05; Table 1).
| Variables | Groups | P Value | ||
|---|---|---|---|---|
| Control (n = 22) | TICBN Blockade (n = 19) | US-Guided ICBN Blockade (n = 19) | ||
| Age | 31.41 ± 7.4 | 29.47 ± 9.7 | 28.9 ± 9.6 | 0.88 |
| Blood pressure (mmHg) | 127.1 ± 9.33 | 119.22 ± 27.4 | 126.4 ± 9.12 | 0.33 |
| Heart rate | 76.45 ± 8.86 | 76.42 ± 9.96 | 74.68 ± 4.84 | |
| ASA | 0.86 | |||
| I | 17 (77.3) | 15 (78.9) | 16 (84.2) | |
| II | 5 (22.7) | 4 (21.1) | 3 (15.8) | |
| Body mass index (kg/m2) | 23.9 ± 1.85 | 22.63 ± 1.8 | 23.05 ± 1.65 | 0.76 |
| Surgery type | 0.24 | |||
| Repair of nerve and tendon surface of left wrist flexion | 3 (13.6) | 2 (10.5) | 4 (21.1) | |
| Wrist serration | 13 (59.1) | 8 (42.5) | 7 (36.8) | |
| Radial nerve explorer and back tendon | 1 (4.5) | 3 (15.8) | 1 (5.3) | |
| Hemi amputation | 1 (4.5) | 0 | 0 | |
| Injury to the forearm and left palm | 1 (4.5) | 0 | 2 (10.5) | |
| Finger repair | 1 (4.5) | 0 | 0 | |
| Multiple finger transplantation and repair Restoration and scarring of the left forearm | 1 (4.5) | 1 (5.3) | 0 | |
| Resection of scarring of forearm | 0 | 0 | 1 (5.3) | |
| Nerve and artery exploration and repair | 0 | 0 | 2 (10.5) | |
| Hand paralysis (transfer to the tendon) | 0 | 0 | 1 (5.3) | |
| Radial flap inverter in place of wrist amputee | 0 | 0 | 1 (5.3) | |
| Hand tendon transfer | 0 | 3 (15.8) | 0 | |
| Finger transplant | 0 | 1 (5.3) | 0 | |
| Median and ulnar nerve repair | 1 (4.5) | 1 (5.3) | 0 | |
| Surgery duration (min) | 115.45 ± 43.2 | 122.11 ± 32.12 | 121.68 ± 28.13 | 0.77 |
| Tourniquet pressure (mm Hg) | 230.4 ± 11.4 | 227.47 ± 10.48 | 222.58 ± 23.69 | 0.49 |
| Active time of the tourniquet (min) | 91.5 ± 25.9 | 109.75 ± 31.18 | 117.1 ± 37.65 | 0.051 |
| Inactive time of the tourniquet (min) | 15.29 ± 1.78 | 13.57 ± 2.42 | 14.87 ± 3.2 | 0.19 |
Abbreviations: ICBN, intercostobrachial nerve; TICBN, traditional intercostobrachial nerve; US, ultrasound.
a Values are expressed as mean ± SD or No. (%).
4.2. Comparison of the Onset and Intensity of Pain Between the TICBN Blockade and Control Groups
The results showed that the mean pain intensity score was significantly lower in the TICBN blockade group (2.65 ± 1.01) than in the control group (6.21 ± 3.16; P = 0.001). Also, the mean time to onset of pain was significantly higher in the TICBN blockade group than in the control group. In other words, pain occurred significantly later in the TICBN blockade group than in the control group (P = 0.021; Table 2).
| Variables | Groups | P Value | |
|---|---|---|---|
| Control (n = 22) | TICBN Blockade (n = 19) | ||
| Pain intensity score | 6.21 ± 3.16 | 2.65 ± 1.01 | 0.001 |
| Time to start pain (min) | 45.28 ± 14.5 | 62.14 ± 21.42 | 0.021 |
Abbreviation: TICBN, traditional intercostobrachial nerve.
a Values are expressed as mean ± SD.
4.3. Comparison of the Onset and Intensity of Pain Between the US-Guided ICBN Blockade and Control Groups
The results showed that the mean pain intensity score was significantly lower in the US-guided ICBN blockade group (2.16 ± 0.96) than in the control group (6.21 ± 3.16; P = 0.001). Also, the mean time to onset of pain was significantly higher in the US-guided ICBN blockade group (65.11 ± 15.33) than in the control group (45.28 ± 14.5; P = 0.013; Table 3).
| Variables | Groups | P Value | |
|---|---|---|---|
| Control (n = 22) | US-Guided ICBN Blockade (n = 19) | ||
| Pain intensity score | 6.21 ± 3.16 | 2.16 ± 0.96 | 0.001 |
| Time to start pain (min) | 45.28 ± 14.5 | 65.11 ± 15.33 | 0.013 |
Abbreviations: ICBN, intercostobrachial nerve; US, ultrasound.
a Values are expressed as mean ± SD.
4.4. Comparison of the Onset and Intensity of Pain Between the US-Guided ICBN Blockade and TICBN Blockade Groups
The results showed no significant difference in the mean score of pain intensity between the US-guided ICBN blockade and TICBN blockade groups (P = 0.48). Also, no significant difference was observed in the meantime to onset of pain between the 2 groups (P = 0.44). Although the mean time to onset of pain was higher in the US-guided ICBN blockade group than in the TICBN blockade group, this difference was not statistically significant (Table 4).
| Variables | Groups | P Value | |
|---|---|---|---|
| TICBN Blockade (n = 19) | US-Guided ICBN Blockade (n = 19) | ||
| Pain Intensity Score | 2.65 ± 1.01 | 2.16 ± 0.96 | 0.48 |
| Time to start pain | 62.14 ± 21.42 | 65.11 ± 15.33 | 0.44 |
Abbreviations: ICBN, intercostobrachial nerve; TICBN, traditional intercostobrachial nerve; US, ultrasound.
a Values are expressed as mean ± SD.
5. Discussion
The axillary block of the brachial plexus is a routine and efficient method for anesthesia of the upper limb during hand and forearm surgery (9). On the other hand, a tourniquet is used to control bleeding in the surgical field. Because part of the inner arm is innervated by the lateral cutaneous branch of the second intercostal nerve (ie, ICBN), despite the axillary block of the brachial plexus, a tourniquet can cause pain that is not tolerable for the patient and disrupts the surgical process. Therefore, it seems that in addition to the axillary block of the brachial plexus, this problem can be overcome by blocking the ICBN. Therefore, in the present clinical trial, in addition to axillary block in patients who were candidates for soft tissue surgery of the hand and forearm, we also blocked the ICBN to examine whether the pain caused by a tourniquet during surgery disappeared. Few studies have been conducted to compare these 2 techniques (conventional vs US) in the ICBN block to evaluate the reduction of tourniquet pain. In 2018, Magazzeni et al. conducted a study on 84 patients who underwent axillary block and divided them into 2 groups (the ICBN block group using US and the block group using the conventional method) (10). They showed that 41 patients (97.6%) who received US-guided block were comfortable with the tourniquet vs 16 patients (38.1%) in the conventional group (10). Their results are completely different from our results. One of the reasons for this difference may be the way the conventional block was done. Considering the use of landmarks in blind blocks without US, the experience and skill of the anesthesiologist can also be effective in the way it is performed and in the results.
Le-Wendling et al. studied 40 patients under US-guided supraclavicular brachial plexus block who received an additional or no ICBN block, with 22 allocated to the intervention group and 18 to the control group (11). They concluded that the presence or absence of ICBN block was associated with the development of tourniquet pain; in addition, the overall incidence of tourniquet pain in the setting of a dense brachial plexus block was low even without ICBN block and even with using tourniquet more than 90 minutes (11). The results of this research are inconsistent with the current study. However, this inconsistency can be explained by considering that the supraclavicular block can make the major part of the upper arm painless.
The present study showed that performing ICBN block in patients who undergo axillary block compared to those who only experience axillary block, in addition to causing a later onset of tourniquet pain, reduces pain intensity. In general, few studies have examined the effects of ICBN block. Intercostobrachial nerve block is not usually used independently as a technique for pain control but is complementary to brachial plexus block (12). The brachial plexus block is widely accepted by anesthesiologists as a RA technique for upper extremity surgeries due to its relative ease and safety (13). Other studies, such as ours, have used ICBN block as a complementary technique to brachial plexus block, especially for anesthesia of the inner surface of the arm and armpit to reduce tourniquet pain (14). It should be noted that there is controversy about the efficacy of ICBN block in controlling tourniquet pain because some researchers showed that the pain caused by the tourniquet was more due to ischemia in the distal area of the closing tourniquet (consequently due to the pressure in this area) than due to the local sensation of the tourniquet closure area (12). Intercostobrachial nerve block is usually performed blindly based on the anesthesiologist’s experience using anatomical nerve pathways. In this method, the upper edge of the biceps muscle is marked in the anterior axillary line; then, LA is injected subcutaneously in the direction of the axillary (inner) surface of the arm downward. In this procedure, both the ICBN and the inner cutaneous nerve of the arm (which connects to the ICBN) are anesthetized (12). Because there are large blood vessels around and in close relation to the ICBN, the traditional method (blind) is associated with a risk of damage to these vessels. Therefore, the use of an accurate method (such as ultrasonography) to determine the ICBN significantly reduces this risk. In this study, the duration of the applied tourniquet was significantly less in the without-block group than in the other 2 interventional groups. This clinically significant difference, as we observed during research, could be due to the pain sensation in some patients in this group and the early deflating of the tourniquet due to their expression of discomfort. However, this difference was not statistically significant (Table 1).
One of the strengths of the present study is that in addition to ICBN block under US guidance, another group of patients was considered in the study design, in which this nerve block was performed in the traditional way (blind). By adding this group, the effectiveness of the ICBN block with or without US guidance was compared. Finally, if there was no difference in the effectiveness of these 2 methods, to increase safety about the possibility of damage to the blood vessels around the ICBN, the blocking method of this nerve was used under US. The results of our study showed that there was no significant difference between the effectiveness of the ICBN block in the traditional group and under US guidance; thus, it is recommended to use US guidance in the block of this nerve. One of the limitations of our study is the single-center nature of this design, which can lead to the generalizability of results. In addition, the small sample size can be considered another limitation of this design. In the present study, matching of the studied groups was not performed, which could be the source of potential selection bias in the study.
5.1. Conclusions
Intercostobrachial nerve block in soft tissue surgery of the hand and forearm, in addition to the axillary block of the brachial plexus, can control the pain caused by tourniquet closure. Since this nerve block under US guidance has the same effect as this nerve block in the traditional way, it is recommended to use US guidance for more safety.