1. Background
Pain is one of the complications after surgery. Unresolved pain after surgery can interfere with sleep and physical activity and lead to poor recovery. These adverse effects may extend the period of delayed discharge rehabilitation and functional recovery (1). Unresolved pain after surgery also has negative effects on the functioning of various respiratory, cardiac, and gastrointestinal systems (2). Thoracic surgery is usually associated with extensive tissue damage after severe surgery. Severe surgical incision pain restricts chest movements and exposes the patient to ventilation-perfusion (V/Q) mismatch, atelectasis, hypoxemia, and infection, resulting in serious problems with the healing process (3, 4).
Epidural anesthesia is one of the most popular methods to deal with postoperative pain. Continuous injection of epidural anesthetics is an effective way to control acute pain after thoracotomy (5). The epidural method allows the patient to walk as soon as possible and accelerates the return of gastrointestinal function. This method is also associated with a reduction in cardiopulmonary mortality in the early postoperative stages (6). However, a single administration of local anesthetics is associated with adverse effects, including hypotension, bradycardia, and muscle weakness (7, 8). In this regard, many drugs with different potency have been used as an adjuvant for local anesthetics, such as morphine, clonidine, ketamine, neostigmine, magnesium, and dexamethasone. Opioids are commonly used for adjuvant therapy (9). However, they are associated with several side effects, including respiratory depression, delayed return of bowel movements, severe itching of the skin, and increased nausea and vomiting after surgery (10).
Dexmedetomidine is approved by the US Food and Drug Administration and provides sedative and analgesic properties to patients without causing respiratory depression (11). It reduces the dose-dependent reduction of epinephrine in plasma as well as the stress response to surgery and intensive care. It can provide intraoperative cardiac support in patients at risk for coronary heart disease (12, 13). It is an α2-adrenoceptor agonist with eight times higher affinity for the α2-adrenoceptor than clonidine (14). It also has fewer hemodynamic side effects due to less stimulation of alpha receptors (15). Dexmedetomidine was initially used as a sedative in intensive care. The unique analgesic properties of dexmedetomidine have led anesthesiologists to use it as preoperative analgesia. Previous studies have shown that dexmedetomidine enhances the analgesic properties of local anesthetics when used in the norepinephrine pathway (16). Unfortunately, some studies have shown that dexmedetomidine alone cannot cause prolonged and sufficient analgesia after major surgery (17, 18).
2. Objectives
This study aimed to evaluate the effect of continuous injection of thoracic epidural dexmedetomidine on analgesia after thoracotomy.
3. Methods
3.1. Study Population
This randomized clinical trial was conducted on adult patients (18 to 70 years old) who were candidates for thoracotomy surgery and had ASA scores of 1 to 3. This study was registered on the Iranian Registry of Clinical Trials website (code: IRCT20181104041550N1). Exclusion criteria were a history of pneumonectomy, arrhythmia, treatment with alpha-adrenoceptor antagonists, epidural catheter placement contraindications (such as coagulopathy, catheter site infection, and vertebral anomalies), no possibility of postoperative extubation (forced expiratory volume in the first second of expiration (FEV1) < 40% or FEV1 > 30% with unsatisfactory V/Q scan and diffusing capacity of lung for carbon monoxide (DLCO) results), underlying diseases (including ischemic heart disease or heart failure, severe valvular heart disease, kidney or hepatic failure, and cirrhosis), hypersensitivity to ropivacaine, dexamethasone, or morphine, history of opioid abuse, receiving routine analgesia, undergoing emergency surgery, and massive bleeding.
Informed written consent was obtained from all patients, and the Ethics Committee of Rasoul Akram Hospital approved the study (code: IR.IUMS.FMD.REC.1399.595).
3.2. Study Intervention
Using a random number table, patients were divided into 2 groups: (1) The ropivacaine group received only ropivacaine before epidural anesthesia; and (2) the ropivacaine + dexmedetomidine group received preoperative epidural anesthesia with a combination of the 2 drugs. Before Epidural catheter insertion, the patients first received 1 L of IV normal saline, then were sedate with midazolam (1 mg) and fentanyl (50 μg) in a sitting position. Under sterile conditions, the 7th to 10th thoracic vertebrae were identified (in the lower extremities of the scapula with the seventh thoracic vertebra). The intersection of the catheter was T6 to T7. If the needle could not enter the space, the catheter was entered at a higher or lower intervertebral space. The needle (18 gauge) was inserted, and 3 mL of lidocaine was injected. The loss of resistance to saline injection was used to confirm epidural space entry. Three milliliters of lidocaine containing 20,000 units of adrenaline was injected to ensure that the needle was not placed into intravascular or intrathecal space. Finally, an epidural catheter was inserted. The drug combination was delivered by an anesthesiologist who was not part of the research team and injected into an epidural catheter. According to the random codes of patients, for the ropivacaine + dexmedetomidine group, 1% ropivacaine (6 mg) with dexmedetomidine (1 mg/mL) was injected as a bolus from an epidural catheter before surgical incision and the pointed order was given as an epidural infusion of ropivacaine and dexmedetomidine during surgery and up to 48 hours after surgery. In the ropivacaine group, only ropivacaine (6 mg) was injected before surgery and continued during surgery and up to 48 hours after surgery. The drug combination was prepared as follows: For the ropivacaine group, 10 mL of 0.5% ropivacaine was mixed with 40 mL of normal saline to obtain ropivacaine with 1% concentration. For the ropivacaine + dexmedetomidine group, 10 mL of 0.5% ropivacaine with 2 mL of dexmedetomidine were mixed with 39 mL of normal saline, and a concentration of 0.1% for ropivacaine and 1 μg/mL for dexmedetomidine was achieved. An infusion pump was used to inject drugs at a rate of 4 mL/h. The patients first received fentanyl (3 to 5 μg/kg) for pre-medication; then, general anesthesia was inducted with propofol (1 to 2 mg/kg) and cisatracurium (0.2 mg/kg). Also, propofol (100 μg/kg/min) and cisatracurium (30 mg) were used to maintain anesthesia until the end of surgery. The patients were then admitted to the intensive care unit (ICU).
3.3. Study Assessments
The rate of sedation was monitored by observing the sedation by determining the Observed Alertness/Sedation Scale (OAAS) score (a score of 3 or above indicates deep sedation). To evaluate neurotoxicity induced by epidural dexmedetomidine, all patients underwent a neurological examination to find any sensory or movement effects during the first week after the operation. Also, the patients were evaluated for postoperative pain using the Visual Analog Scale (VAS) during recovery and 2, 6, 12, 24, 36, and 48 hours after surgery. For the patients with a pain score greater than 3, morphine (0.1 mg/kg) was slowly injected intravenously. The possibility of epidural catheter replacement for patients was evaluated. To determine the efficiency of patients’ ventilation, arterial blood gas (ABG) was evaluated to determine the level of PaCO2. The patients who had respiratory failure or needed intubation after surgery were recorded. In the present study, the patient and outcome assessor were unaware of the type of intervention (double-blind). In the case of dexmedetomidine-related side effects, the injection would be stopped. In the case of nausea and vomiting, 1 mg of ondansetron was injected intravenously. In the case of shivering, 10 mg of meperidine was injected intravenously.
3.4. Statistical Analysis
For statistical analysis, results were presented as mean ± SD for quantitative variables and frequency (percentage) for categorical variables. Continuous variables were compared using the t test or Mann-Whitney test whenever the data did not appear to have a normal distribution or when the assumption of equal variances was violated across the study groups. The chi-square test or Fisher exact test was used to compare the categorical variables. P values of ≤ 0.05 were considered statistically significant. Data were analyzed using SPSS version 23 (SPSS Inc, Chicago, IL, USA).
4. Results
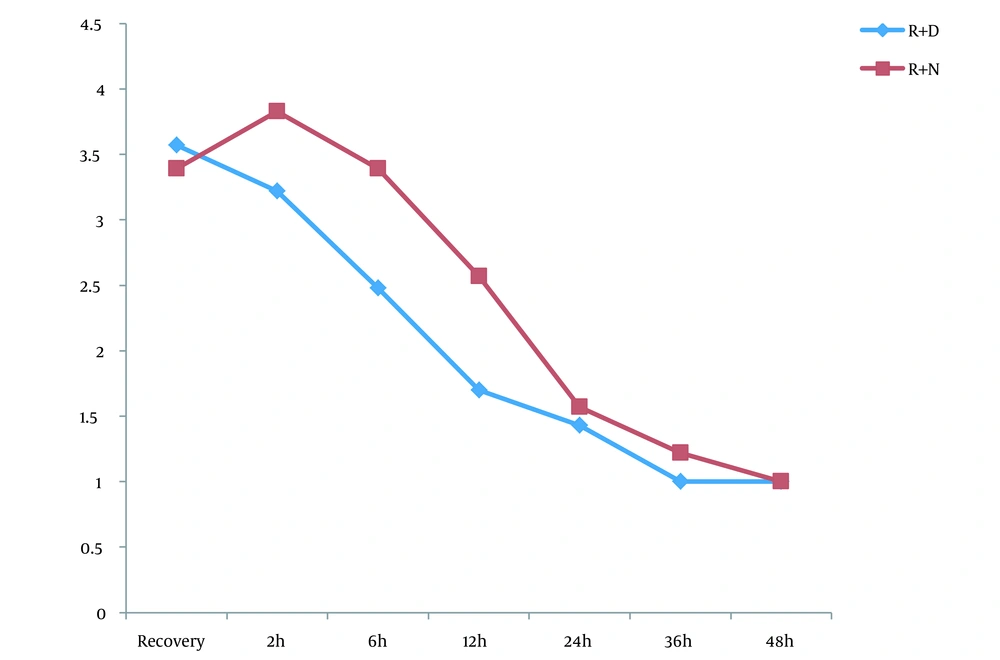
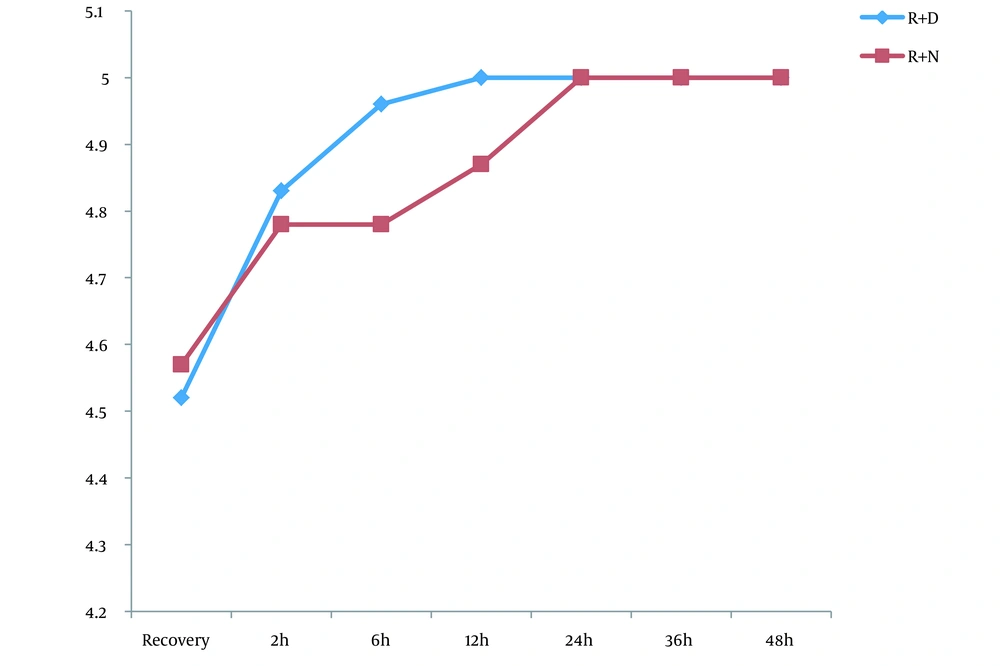
Of the 46 patients, 23 were scheduled for a ropivacaine + dexmedetomidine regimen and 23 for ropivacaine alone. The 2 groups were similar in baseline characteristics of male gender (52.2% vs. 52.2%; P = 1.000), mean age (46.39 ± 13.34 years vs. 48.39 ± 13.69 years; P = 0.626), and mean body mass index (23.03 ± 3.75 kg/m2 vs. 23.40 ± 2.87 kg/m2; P = 0.712). The mean pain score was similar at recovery and 2 hours after the assessment of pain score at the 6th to 36th hours; however, it showed a lower pain score in the ropivacaine + dexmedetomidine group than in the ropivacaine group (Figure 1). Regarding the OAAS sedation score, comparing the mean OAAS score indicated no difference between the 2 study groups; however, a gradual increase in both groups was evident in terms of sedation scores (Figure 2). In the 2 groups of injecting ropivacaine with and without dexmedetomidine, the rate of morphine administration after surgery was 43.4% and 65.2%, respectively, indicating no difference between the 2 groups (P = 0.139). However, the first group received significantly lower doses of morphine after the end of the study (3.26 ± 0.90 mg vs. 7.04 ± 1.48 mg; P = 0.035).
5. Discussion
Pain after major surgery is one of the most annoying complications, which, sometimes due to its chronic and prolonged nature, may disrupt daily life activities, induce mood disorders (such as anxiety and depression), and even impair quality of life. Various pharmacological methods and invasive non-pharmacological modalities (such as nerve root block) have been used to relieve pain after thoracotomy surgery. The use of opioid drugs is limited due to hemodynamic disorders after surgery; therefore, mainly, drug-based methods are effective and safe with minimal hemodynamic side effects. Recently, the use of epidural dexmedetomidine to relieve pain from major surgeries (such as thoracotomy) has been studied, and its effects have been compared with other drugs, such as opioids, nonsteroidal anti-inflammatory drugs (NSAIDs), fentanyl, and amino-amide groups. This study aimed to evaluate and compare the analgesic effects of dexmedetomidine in patients undergoing thoracotomy. This clinical trial enrolled 46 patients and randomly assigned them to dexmedetomidine in combination with ropivacaine or ropivacaine alone (as a control group). The changes in pain scores (based on VAS) and sedation scores (based on OAAS), as well as the need for analgesic (morphine) administration within 48 hours after surgery, were compared between the 2 groups.
The results showed that the mean pain of patients 6 - 48 hours after surgery was much lower in the ropivacaine + dexmedetomidine group than in the ropivacaine group. However, the sedation score did not show a significant difference between the 2 groups. Also, the mean dose of morphine requested after surgery was significantly lower in the ropivacaine + dexmedetomidine group than in the ropivacaine group. Accordingly, the present study provided sufficient evidence for the greater effectiveness of the ropivacaine-dexmedetomidine combination.
Almost all studies evaluating the effectiveness of dexmedetomidine in relieving thoracotomy pain have acknowledged its superiority over other protocols. In the study by Li et al. (19), the group treated with dexmedetomidine had lower pain scores, lower neuropathic pain incidence, and lower levels of tumor necrosis factor α and interleukin-1β cytokine than the placebo group. Also, the prescribed dose of opioids and the number of opioid administrations were significantly lower in the group receiving dexmedetomidine than in the normal saline group. In a study by Mao et al. (20), dexamethasone was not associated with a reduction in the number of postoperative analgesic requests. Although in their study, there was no difference in the severity of postoperative pain, duration of hospitalization, chronic pain, or quality of life between patients in the 2 groups of dexmedetomidine and placebo, dexmedetomidine administration was associated with improved postoperative sleep quality. In the study by Choi et al. (21), the pain score in the dexmedetomidine group was significantly lower than in the placebo group during the first 48 hours after surgery. The total dose of postoperative opioids was significantly lower in the dexmedetomidine group than in the normal saline group. Overall, the level of patient satisfaction was much higher in the dexmedetomidine group than in the placebo group. Finally, in the study by Yan et al. (22), the VAS score during the 6 to 48 hours after surgery was much lower in the ropivacaine + dexmedetomidine group than in the ropivacaine group (22), which is completely consistent with our study.
As one of the potential limitations of the present study, changes in hemodynamic parameters were not examined and compared in our study, while in some studies, significant induction of bradycardia and hypotension in the group receiving dexmedetomidine was mentioned (23, 24); however, in some studies, no difference was observed between the 2 groups with or without dexmedetomidine in terms of changes in hemodynamic parameters, even heart rate and blood pressure (25). Further studies with larger sample sizes and different doses of dexmedetomidine are needed to determine the optimal dose and evaluate simultaneous changes in hemodynamic conditions.
5.1. Conclusions
The administration of dexmedetomidine with ropivacaine resulted in greater pain relief within 48 hours after thoracotomy than ropivacaine alone. Also, following the administration of the first medication regimen, the dose of analgesic used after surgery was significantly reduced.