1. Introduction
Diabetic polyneuropathy (DPN) occurs in 20% - 30% of patients (1), and it is defined as a distal, symmetric polyneuropathy with sensory symptoms, including pain, paresthesias, burning, allodynia, and hyperalgesia (2); also, it is described as motor and autonomic neuropathy.
The pathogenesis is not fully understood, but DPN seems to be related to more than one mechanism.
Nerve damage is mainly due to hyperglycemia and insulin resistance, leading to alteration of the
metabolic pathway of polyol-sorbitol with functional alteration of the intracellular Na/K pump. Increased free radicals and hyperactivation of glycation pathways contribute to oxidative stress of nerves and vasa nervorum, resulting in microvascular abnormalities (3).
The diagnosis of DPN is made after the exclusion of other neuropathies, autoimmune disorders (such as Sjogren syndrome, lupus, and rheumatoid arthritis), inflammatory disorders (such as chronic inflammatory demyelinating polyneuropathy (CIDP)), infectious disorders (such as HIV and hepatitis B and C), and inherited disorders (such as Charcot-Marie-Tooth disease), as well as factors such as cancer, dysvitaminosis, hypothyroidism, alcoholism, or nerve damage by injury or entrapment. The American Diabetes Association strongly recommends early screening repeated at defined time intervals. According to the Toronto DPN international consensus, a definite diagnosis requires at least 1 symptom and/or at least 1 sign of neuropathy and abnormality in nerve conduction study (NCS) (4). The recently introduced high-resolution ultrasonography (HRUS) of peripheral nerves with the evaluation of cross-sectional area (CSA) has an increasingly important role in this regard. This is a non-invasive, easily reproducible, and cost-effective examination, allowing us to make an early diagnosis that can potentially detect subclinical forms. It is also valid for assessing the degree of nervous impairment based on ultrasound patterns (5).
Currently, there are no specific treatments for DPN. Therapeutic goals include symptom management and behavioral changes to slow disease progression (6). The pharmacological pain management of painful DPN (P-DPN) includes gabapentinoids, serotonin-norepinephrine reuptake inhibitors, tricyclic antidepressants, and opioids (7). However, adherence is poor due to both inadequate pain control and significant side effects, and within 6 months, more than 60% of patients discontinue it (8, 9).
Spinal cord stimulation (SCS) has been proposed for the treatment of patients with P-DPN who do not respond to conventional medical treatment or with poor drug compliance (10). The most frequent SCS modalities used were low-frequency paresthesia and high-frequency SCS (10 kHz). The aforementioned neurostimulation could lead to significant pain relief at 1 and 3 months (11).
The fast-acting sub-perception therapy (FAST) modality is a novel non-paresthetic stimulation that has unique features, requires significantly less energy delivered than previously reported for subthreshold stimulation at 1 - 10 kHz, has the possibility to perform intraoperative setting trials, and can rapidly reduce pain (within a few minutes) (12).
The aim of this study was to report the case of a patient with severe P-DPN who had immediate pain relief after implantation of SCS in the FAST modality, with constant pain relief at 3 months. We have also evaluated the comparative CSA of the most involved nerves, namely, external popliteal nerve (EPN), posterior tibial nerve (PTN), and sural nerve (SN) by preoperative and postoperative HRUS.
2. Case Presentation
A 58-year-old female patient was referred to our hospital in 2020 (Civitavecchia, Italy). The patient had been suffering from diabetes since 2009. The patient was being treated with semaglutide, 0.25 mg sc. For the previous 3 years, her clinical history was complicated with P-DPN, symmetrically affecting feet and legs with “stocking distribution,” characterized by tingling, numbness, burning pain, hypoesthesia, allodynia, and severe sharp pain with a Numerical Rating Scale (NRS) of 9 and Douleur Neuropathique en 4 (DN4) of 7. No motor deficits or abnormal deep tendon reflexes were detected. Neither dysvitaminosis nor autoimmune/rheumatological disorders were present in anamnesis, and both were excluded by specific blood tests. The impact of severe pain on her quality of life was considerable. The patient failed conservative therapeutic options and medications of the first and second line for the treatment of P-DPN. The patient was elected to the all-in-one SCS implantation technique to reduce the risk of infection in the FAST modality. Preoperative tests, dorso-lumbar magnetic resonance imaging (MRI), and lower-limb somatosensory evoked potentials were normal. Electromyography (EMG) detected no signs of radiculopathy, and electroneurography (ENG) showed a reduction of sensory conduction velocity (SCV) of the SN (35 m/s) bilaterally with normal amplitude. Before surgery, the HRUS of peripheral nerve CSA was performed by an examiner with more than 10 years of experience in nerve ultrasonography using a linear probe (18 MHz; Samsung RS80A Ultrasound Machine). The cross-sectional area was assessed by tracing the inner margin of the hyperechoic epineurium stroma, with the transducer perpendicular to the nerve. Cervical roots and upper limb nerve CSA measurements were C7 = 0.10 cm2, C6 = 0.095 cm2, ulnar nerve (UN) = 0.063 cm2 at forearm, median nerve (MN) = 0.089 cm2 at forearm, and superficial radial nerve (SRN) = 0.023 cm2 at forearm. Further, lower limb measurements were: (1) EPN (3 cm proximal to the base of the fibular head; left leg = 0.36 cm2; right leg = 0.38 cm2); (2) PTN (in the retromalleolar region, 3 cm proximal to the apex of the tibial malleolar process; right and left legs (2 measurements per side) = 0.19 cm2); and (3) SN (3 cm proximal to the retromalleolar region next to the small saphenous vein = 0.06 cm2). The patient was assessed before and after surgery using NRS, Short Form-36 Health Survey (SF36), Brief Pain Inventory (BPI), and Pain Interference Score (PIS).
After obtaining written consent, SCS implantation was performed under local anesthesia and intravenous light sedation. An octopolar lead (SC-2218-50 Linear Boston Scientific Neuromodulation Corporation, Valencia, CA 91355, USA) was introduced into the posterior epidural space through the L1 - L2 interlaminar space under fluoroscopic guidance. The lead was positioned with the tip at T9 at the level of the dorsal columns after adequately covering (more than 80%) with paresthesias in the painful area. The FAST stimulation was set with the following parameters: A frequency of 90 Hz and pulse width of 210 ms, cycled on/off 1/1. The lead was then fixed by the anchor to the thoracolumbar fascia and then tunneled to the left upper buttock pocket to connect to the implantable pulse generator (WaveWriter ALPHA PRIME 16, Boston Scientific Neuromodulation Corporation, Valencia, CA 91355, USA). After 2 days, the patient was discharged with an NRS of 1. No complications occurred.
3. Results
In our patient, SCS implantation with the FAST modality resulted in significant pain relief, starting from the immediate postoperative period and up to a 3-month follow-up period. At 1 month follow-up, NRS values significantly decreased from 9 to 1, BPI showed a significant improvement, PIS decreased from 9.7/10 to 0 (-100%), DN4 decreased from 7 to 0, and the patient completely discontinued antalgic pharmacological therapy previously assumed.
The patient also had a significant improvement in all items of the SF36 in both physical component summary (preoperative score (PCS) = 19.08 increased to postoperative = 57.08 of +199%) and mental component summary (from preoperative (MCS) = 16.92 to postoperative = 58.87 of +248%).
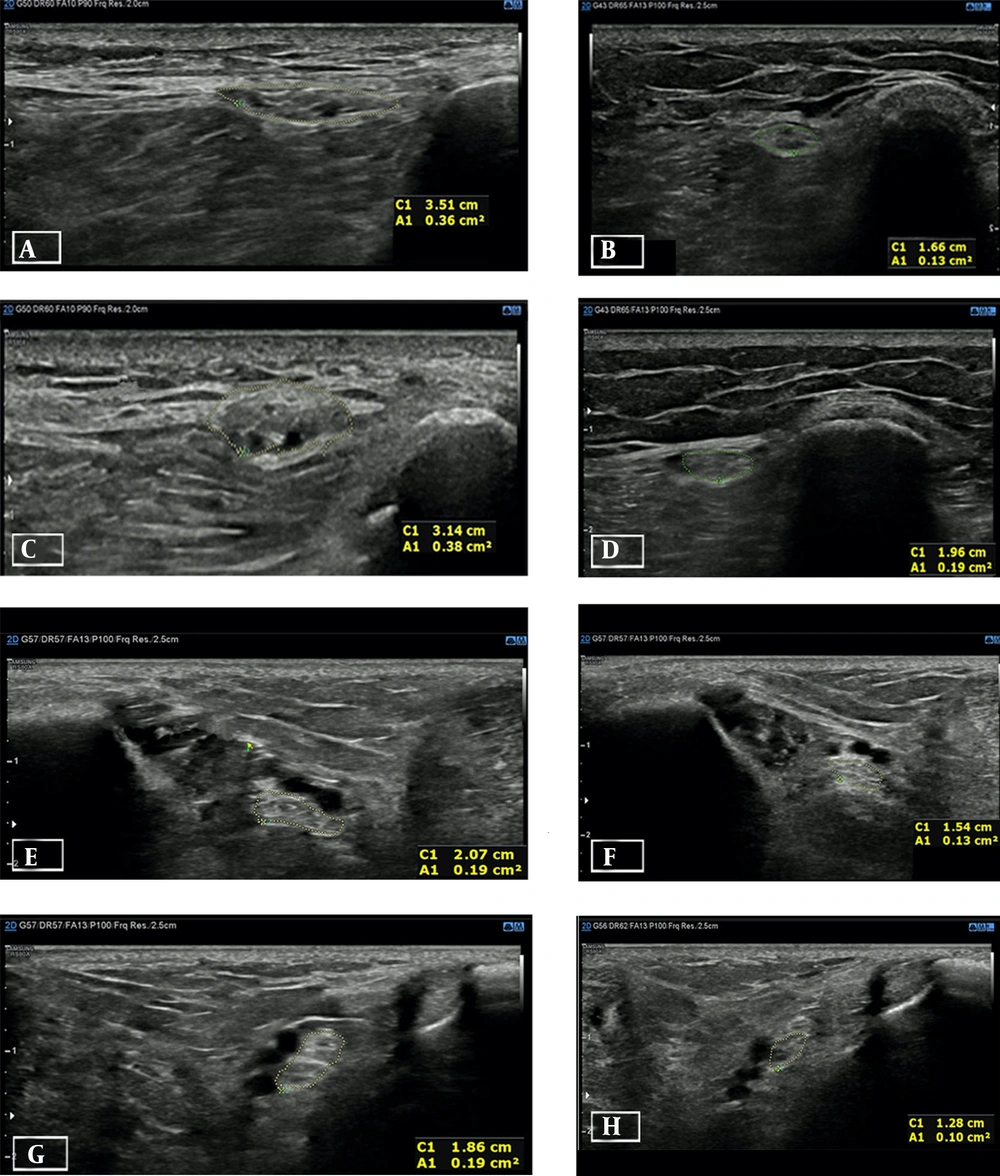
Very interesting data were provided by the HRUS of peripheral nerves by CSA measurement, repeated 1 and 2 months after implantation (Figure 1). On ultrasound examination, we found a reduction of CSA in all the nerves involved in our case, namely, EPN, PTN, and SN (Table 1). Statistical analysis was performed by comparing preoperative CSA measurement values with 1- and 2-month follow-ups, respectively, and ended up with statistically significant differences from preoperative values and follow-up as described. In the first analysis (1-month follow-up), the CSA of the 3 nerves decreased (from mean = 0.206 and SD = 0.139 (preoperative period) to mean = 0.118 and SD = 0.74 (1-month follow-up period)); a paired t test showed a statistically significant reduction = 0.08 (95% CI, 0.16 - 0.016 with t[5] = 3.164; P = 0.026; P < 0.05; Wilcoxon non-parametric test: P = 0.027; P < 0.05). In the second analysis (2-month follow-up), a paired-sample t test was performed to compare preoperative CSA measurements (mean = 0.207 and SD = 0.14) with 2-month follow-up (mean = 0.102 and SD = 0.627), and it showed a statistically significant reduction of CSA values = 0.105 (95% CI, 0.19 - 0.015 with t[5] = 3.017; P = 0.03; P < 0.05; Wilcoxon non-parametric test: P = 0.027; P < 0.05).
The high-resolution ultrasonography (HRUS) of the external popliteal nerve and posterior tibial nerve, as well as the preoperative and 2-month follow-up results of the left and right legs. The dotted yellow line indicates the cross-sectional area (CSA) of any nerve expressed in cm2 at A1 in each image. A and B refer to the left external popliteal nerve (EPN), A, preoperative period; B, at 2-month follow-up; C and D refer to right EPN; C, preoperative period; D, at 2-month follow-up; E and F refer to the left posterior tibial nerve (PTN); E, preoperative period; F, at 2-month follow-up; G and H refer to right PTN; G, preoperative period; H, at 2-month follow-up
| Nerve | Right Lower Limb | Left Lower Limb | ||||||
|---|---|---|---|---|---|---|---|---|
| Preop (cm2) | Fu 1 Month (cm2) | Fu 2 Month (cm2) | Delta % | Preop (cm2) | Fu 1 Month (cm2) | Fu 2 Month (cm2) | Delta % | |
| EPN | 0.38 | 0.2 | 0.19 | -50 | 0.36 | 0.19 | 0.13 | -63.9 |
| PTN | 0.19 | 0.12 | 0.10 | -47.37 | 0.19 | 0.14 | 0.13 | -31.6 |
| SN | 0.06 | 0.03 | 0.03 | -50 | 0.06 | 0.03 | 0.03 | -50 |
Abbreviations: Preop, preoperative; FU 1 month, 1-month follow-up; FU 2 month, 2-month follow-up; EPN, external popliteal nerve; PTN, posterior tibial nerve; SN, sural nerve.
ENG was also performed at the 1-month follow-up and showed increased sural nerves SCV: left = 58.3 m/s, right = 64.3 m/s.
At the 3-month follow-up, the patient’s pain relief was stable, and there were no statistically significant changes in the score of the questionnaires administered, as well as in the measurement of CSA in all the nerves involved. No changes in the stimulation settings were necessary.
4. Discussion
As for many other chronic pain syndromes, early diagnosis and treatment are key to improving clinical outcomes in patients with P-DPN, but there is still no gold standard treatment. The introduction of HRUS is viewed with increasing interest in both diagnosis and follow-up, as it can identify the extent of nerve damage through the analysis of size, blood vessels, echo, and mobility of the nerve, earlier than EMG, ENG, and nerve conduction tests (13).
The analysis and study of peripheral nerves in subjects with P-DPN by HRUS can help us describe the severity of the clinical form and recognize atypical or subclinical forms, as it can identify the extent of nerve damage through the analysis of size, blood vessels, echo, and mobility of the nerve (13).
Many studies have shown that the enlargement of CSA in these patients is frequent, as well as the alteration of the ultrasonographic pattern, although validated scores for result standardization are still lacking. Currently, reference is made to the Bochum ultrasound score (14) and the ultrasound pattern sum score (UPSS) (15).
However, it has never been demonstrated, at least so far, that these patterns of nerve damage can be reverted, as happened in our case, in which the clinical benefit obtained by choosing to implant an SCS in the FAST modality, led to the reduction of CSA in the nerves most affected.
SCS seems to improve the microcirculation of the stimulated nerves, and this may restore, at least in part, the typical arteriosclerotic damage that occurs in diabetic patients on small caliber vessels such as vasa nervorum (16).
Other pathogenetic mechanisms involved in P-DPN are cytokine inflammatory downstream and intracellular edema due to reduced function of the Na+/K+ pump and Na+ retention of intracellular fluid, increased oxidative stress with consequent mitochondrial damage, resulting in neuronal apoptosis and demyelination. We can hypothesize that the marked reduction of CSA observed in the peripheral nerves of the lower limbs may be somehow linked to the reduction of edema and water content of the nerves, being so early, 1 month after implantation. The clinical outcome of this treatment, both for the reduction of perceived pain and the improvement of the quality of life, should make us reflect on the possible therapeutic effect of SCS in patients with P-DPN. In this regard, can we hypothesize that SCS could have a therapeutic role rather than a symptomatic one? Can we hypothesize a correlation between a reduction in painful symptoms with the implantation of SCS, the improvement highlighted by the instrumental examinations performed, and the significant change in the score of the questionnaires administered? This clinical case could make us reflect on the fundamental role that SCS may have in the treatment of DPN from a symptomatic point of view but, above all, from a therapeutic point of view.
We think SCS could be a valid and effective tool in patients with severe P-DPN. The use of HRUS has allowed us to objectify the results obtained on peripheral nerve ultrasound analysis, already 1 month after SCS. What can be suggested from these data has brought us to speculate a therapeutic role of SCS in the FAST modality for severe DPN. The limit of this case report is the short follow-up period, but we will continue to follow the patient over time. However, we recommend a feasibility study on the topic with a larger sample and a longer follow-up. We would also like to encourage the use of HRUS not only in diagnosis but also in prognostic assessment in patients with P-DPN. We are aware that studies with larger patient samples will be needed, but we believe this case may be a starting point for further studies.