1. Introduction
The majority of symptomatic patients affected by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) show flu-like symptoms (1). However, due to the neurotropic property of the virus (2), neurologic manifestations, such as myalgia (3, 4), headache (5), anosmia and ageusia, ataxia, seizures, are common and may be the early signs and symptoms of coronavirus disease 2019 (COVID-19) (2). However, morbidity and mortality increase with more severe neurologic complications (6). Complications, such as encephalopathy, meningoencephalitis, Guillain-Barré syndrome, acute disseminated encephalomyelitis, stroke, epilepsy, chemosensory disorders, as well as neurological and muscular disorders, have been reported in patients with COVID-19 (7).
Currently, available data for COVID-19 do not indicate that pregnant individuals are at increased risk of infection (8), but the risk of severe disease and mortality is high in this patient population (9, 10). The severity of COVID-19 can vary widely during pregnancy, with about 85% of women having a mild illness and 15% having severe to acute disease. The most commonly reported symptoms are fever, cough, shortness of breath, and diarrhea (8). Herein, we report a pregnant woman in her 10th gestational week presented with neurological impairment and a reduced state of consciousness with a positive polymerase chain reaction (PCR) test for SARS-CoV-2 on cerebrospinal fluid (CSF) before positive SARS-CoV-2 PCR for a nasopharyngeal swab.
2. Case Presentation
A 10-week pregnant 32-year-old female (gravida 3, para 1, abortion 1) was presented to the emergency department of Firoozabadi hospital, Tehran, with sudden impairment in walking, balance, speech, and consciousness that had started the night before. A review of her history revealed a seven-day history of fever, chills, myalgia, and general weakness before admission. The patient also had a history of close contact with a confirmed case of COVID-19 (i.e., her spouse) two weeks prior to the presentation. She had not been vaccinated against SARS-CoV-2. Past medical history was significant only for an episode of febrile seizure at the age of eight years.
An initial physical exam revealed a temperature of 36.8°C, heart rate of 74 beats/min, respiratory rate of 20/min, blood pressure of 112/68 mmHg, a height of 160 cm, weight of 76 kg, body mass index of 29.6 kg/m2, and peripheral oxygen saturation of 97% in room air. On neurological examination, the patient was confused and had limited eye contact, but the time, place, and person orientations were intact. Other findings on examination were horizontal nystagmus, ataxia, reduced muscle force of the lower limbs (2/5), and positive bilateral Babinski sign. Trans-abdominal ultrasonography revealed a fetus of 10 weeks gestation without intra-abdominal free fluid or any abnormality in the peritoneal cavity.
Laboratory investigation of blood specimen was unremarkable except for an elevated lactate dehydrogenase (LDH) level (456 IU/L), erythrocyte sedimentation rate (37 mm/h), and C-reactive protein level (340 mg/dL) (Table 1). The nasopharyngeal swab sample PCR test for SARS-CoV-2 was negative on the first admission day. A lumbar puncture was also performed on the same day in the emergency department. The CSF was clear and colorless; microscopic examination revealed < 5 white blood cell count (WBC)/L with 1000 red blood cell count (RBC)/L. The protein level was 121 mg/dL, and LDH was equal to 70 IU/L. No organisms were observed on the Gram stain, and the result of the CSF culture was negative as well. The CSF PCR testing for cytomegalovirus was also negative, but CSF testing for SARS-CoV-2 reverse transcription PCR (RT-PCR) was positive.
| Variables | Values |
|---|---|
| Hematology | |
| RBC, cells/L | 3,900,000 |
| Hemoglobin, g/dL | 10.9 |
| MCV, fL | 92.5 |
| MCH, pg | 27.9 |
| MCHC, % | 30.19 |
| WBC, /µL | 5,100 |
| Neutrophils, % | 60 |
| Lymphocytes, % | 33.1 |
| Platelets, /µL | 147,000 |
| CRP, mg/dL | 340 |
| ESR, mm/hr | 37 |
| Blood Biochemistry | |
| BUN, mg/dL | 5 |
| Creatinine, mg/dL | 0.6 |
| Na, mmol/L | 140 |
| K, mmol/L | 3.8 |
| AST, IU/L | 24 |
| ALT, IU/L | 17 |
| ALP, IU/L | 121 |
| Blood sugar, mg/dL | 70 |
| Ferritin, ng/mL | 192 |
| LDH, IU/L | 456 |
| Venous Blood Gas (VBG) | |
| PaCo2, mmHg | 41.3 |
| HCO3, mEq/L | 21.3 |
| PH | 7.32 |
| BE | -4.7 |
| CSF Analysis | |
| Appearance | Clear/colorless |
| RBC | 1000 |
| Glucose, mg/dL | 51 |
| Protein, mg/dL | 121 |
| LDH, IU/L | 70 |
| CSF Culture | No growth |
Abbreviations: CSF, cerebrospinal fluid; RBC, red blood cell count; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; WBC, white blood cell count; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; BUN, blood urea nitrogen; Na, sodium; K, potassium; AST, aspartate aminotransferase; ALT, alanine transaminase; ALP, alkaline phosphatase; LDH, lactate dehydrogenase; PaCO2, partial pressure of carbon dioxide; HCO3, bicarbonate; VBG, venous blood gas.
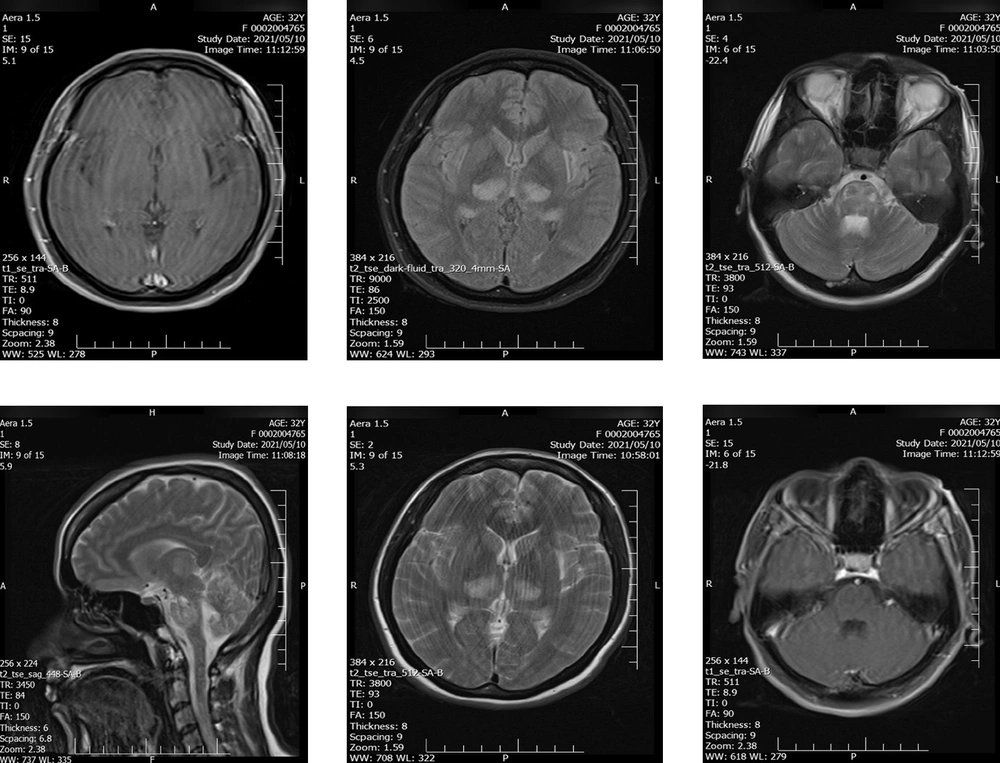
Axial brain magnetic resonance imaging (MRI) revealed a large altered signal area in bilateral symmetric areas of the thalamus, cerebral peduncles, and brainstem, including the midbrain and pons. These findings reflect the involvement of the spinothalamic tract, which was presented as high signal intensity on T2-weighted-fluid-attenuated inversion recovery (T2-FLAIR), suggestive of an acute inflammatory process (Figure 1).
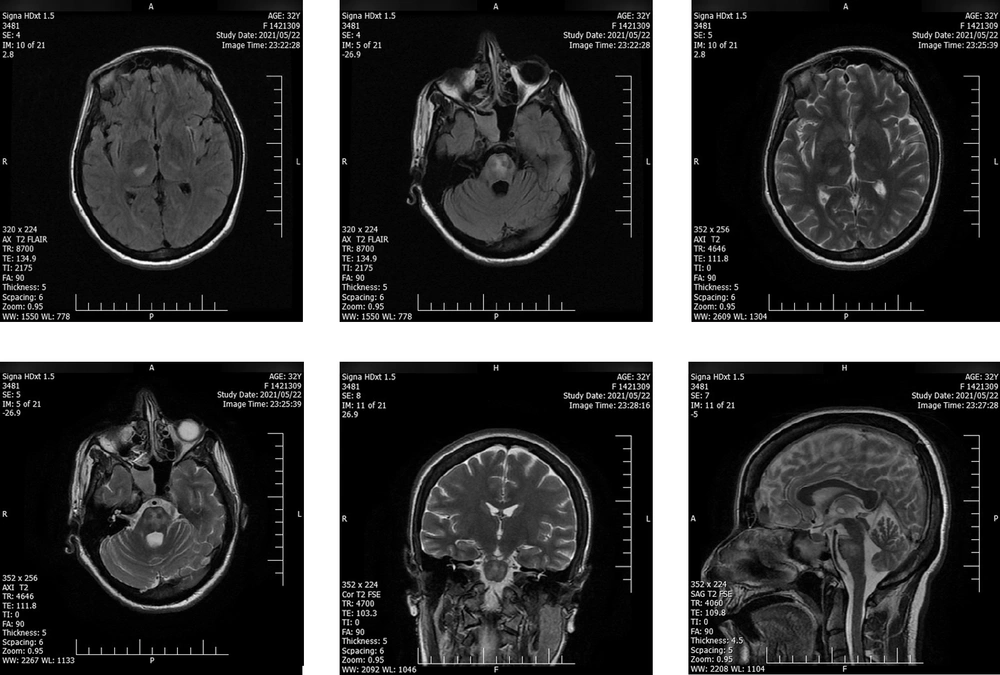
Intravenous immunoglobulin (IVIG) (20 gr/day) was administrated and continued for six days in combination with daily enoxaparin (40 mg subcutaneous), daily methylprednisolone (1 gr/day for seven days), levetiracetam (50 mg/kg twice daily), and daily remdesivir (200 mg on the first day and then 100 mg/day IV for five days). The second consecutive nasopharyngeal RT-PCR test on the second day of admission was positive for SARS-CoV-2. Except for lower limb muscle strength, the patient's neurological condition gradually recovered, and she was discharged from the hospital on the 10th day of admission. The patient was able to walk with assistance at the time of discharge. Two days after discharge, a second MRI showed decreased signal hyper-intensity on T2- FLAIR (Figure 2). After six months of follow-up, the recovery of muscle forces was minimal. However, the control MRI, taken simultaneously, did not demonstrate any abnormality or remaining inflammatory signs. A term neonate was born with a normal delivery at 37 weeks of gestational age, weighing 3200 gr and a height of 50 cm. The newborn was vigorous at the time of delivery with a normal appearance.
3. Discussion
The neurologic features of COVID-19 could be due to the direct effects of the virus on the nervous system, para-infectious or post-infectious inflammation of the nervous system and vascular compartment, or nonspecific complications of systemic disease (11). Neurological complications have been reported in up to 36% of hospitalized COVID-19 patients (12). Nevertheless, encephalitis is not a common complication of SARS-CoV-2 and has been observed more commonly as a para-infectious manifestation that occurs within days to 2 weeks of the primary infection (13). The diagnosis of COVID-19 in pregnant women is similar to non-pregnant individuals (12). Notably, most pregnant women with SARS-CoV-2 infection are asymptomatic or only have mild symptoms. However, it has been proven that they are at an increased risk for hospitalization, requirements for mechanical ventilation, intensive care unit admission, mortality, and preterm labor or stillbirth (9, 10). Furthermore, it has been postulated that SARS-CoV-2 infection can be associated with ischemic injury of the placenta due to vascular damage. The maternal inflammatory response to the virus may also deleterious impact on the offspring. Cytokine response occurs in both maternal and fetal circulation. However, the responses are variable, and different interleukins are increased on each side. A reduced number of T-cells and alteration in T-cell subsets may compromise materno-fetal tolerance (14).
The patient was admitted to the hospital with decreased consciousness, positive Babinski sign, and reduced force of lower limbs. Initial physical exam brought up some differential diagnoses, such as herpetic or cytomegalovirus encephalitis, a brain tumor, pseudotumor cerebri, and intracranial hemorrhage, as complications of eclampsia, which was ruled out by laboratory results and brain magnetic resonance venography. According to the findings of brain MRI, bilateral thalamus and pons hyper-signaling without enhancement, acute disseminated encephalomyelitis (ADEM), and acute necrotizing encephalitis (ANE) were included in differential diagnoses. To rule in ADEM, which was not definite, we expected thalamus enhancement in MRI and the presence of WBC in CSF. Another strong suspicion was ANE with the involvement of the brain stem. A COVID-19 case with such a diagnosis was also reported in a patient with aplastic anemia in September 2020 (15). In that case, the ANE was rapidly progressive with seizures, reduced consciousness, and vomiting that occurred 12 - 72 hours after the onset of viral infection symptoms. Treatment in the mentioned case was unsuccessful; the patient showed no response to steroid therapy and died on the eighth day of admission. This was contrary to what happened to our patient. The diagnosis for COVID-19 was confirmed with positive COVID-19 RT-PCR. Furthermore, the patient responded to medical treatment such that she regained consciousness. Most of the symptoms and signs were regressed, except for the mild weakness in the lower limb and the new onset of palatal myoclonus. The encephalitis was assumed most likely to represent an immune-mediated phenomenon as the patient's condition improved following IVIG, corticosteroid therapy, and other medical treatments. Fortunately, a normal appearance term neonate was born without adverse consequences.
Although there are few reports of positive SARS-CoV-2 PCR tests from CSF samples (16, 17), this case was positive one day before the nasopharyngeal sample became positive for the virus. Salman et al., in a systemic review of 14 confirmed cases of COVID-19, reported that in 21.4% of patients, the nasopharyngeal sample was negative for SARS-CoV-2 at the time the CSF was positive for the virus (16). Not all the cases in the latter review had a severe neurological involvement; however, 28.6% of the patients were admitted to the Intensive Care Unit, and 14.3% died. It can be interpreted that a positive CSF test for SARS-CoV-2 is not associated with the severity of the disease, nor can it determine the prognosis of the disease, and the presence of other clinical and para-clinical findings may predict the disease outcome.
3.1. Conclusions
Atypical neurologic manifestations of COVID-19 in a pregnant woman without flu-like symptoms and a negative PCR test of the nasopharyngeal sample can make the diagnosis difficult. Therefore, meticulous history taking and further laboratory and MRI evaluation may result in proper diagnosis and appropriate treatment. The CSF samples in such patients may be positive for SARS-CoV-2 in PCR, while the nasopharyngeal sample is negative. Neurologic involvement of SARS-CoV-2 may result in ANE, which in this case was responsive to IVIG and corticosteroid therapy.