1. Background
Facemasks are a critical component of personal protective equipment, especially for healthcare workers. When such workers faced the spread of the novel pathogen SARS-CoV-2 (COVID-19 virus), there was a lack of scientific data regarding the physiological effects of facemasks.
The World Health Organization (WHO) proposed the extensive use of facemasks by individuals experiencing symptoms of COVID-19 and the general use of facemasks in, for example, crowded places. It particularly makes sense for healthcare workers who come into direct contact with patients and work for long periods in enclosed environments (1-3). However, uncertainties remain regarding how best to use surgical masks and ventilators to protect healthcare workers treating COVID-19 patients, PTS, etc. Several healthcare organizations have published studies on whether and to what extent surgical masks offer sufficient protection from COVID-19 compared to N95 masks. The studies (4) indicate that N95 masks, which filter out approximately 95% of small particulate substances from the air (including the COVID-19 virus), make breathing difficult for the person wearing the mask. These masks could reduce oxygen intake by 5 - 20%, which may be an important reduction rate (5). There are several warning signs of reduced oxygen intake, including vertigo and lightheadedness; such symptoms affect the performance of healthcare workers, particularly those who must wear masks for many hours while working in clinics (6).
Measuring cerebral oxygenation constitutes a noninvasive technique that allows for the constant assessment of tissue oxygenation. Cerebral oximeters can determine cerebral regional oxygen saturation (rSO2) in the brain and represent an index of cerebral oxygenation. The supply of oxygen to the brain can be affected by various physiological variables, including blood oxygenation, cardiac output, pulmonary function, blood pressure, hemoglobin, and vasoactive, all involved in regulating cerebral oxygenation. Modifying most of these physiological processes changes an individual's rSO2 (7). Both cerebral and pulse oximeters use light transmission and absorption to quantify the ratio of oxygenated to deoxygenated hemoglobin in cerebral tissue (2). However, there is a difference between these two kinds of oximetry (8), as cerebral oximetry seems to be more sensitive and accurate than pulse oximetry (9). The measurement of tissue oxygen saturation by cerebral oximetry reflects the saturation of hemoglobin in cerebral tissue (10) and the equilibrium between oxygen supply and demand. Pulse oximetry measures an individual's oxygen supply rather than the delivery of oxygen (11, 12).
Assessing cerebral oxygenation limits postoperative injury and mortality disruption by indicating problematic oxygenation at the systemic and tissue levels (13). Maintaining continuous information related to cerebral blood and oxygen is vital, as it can offer increased protection (14).
It has been hypothesized that the use of masks may cause hypoxia and affect cerebral oxygenation. The present study investigated whether cerebral oxygen saturation, as measured in hemoglobin and end-tidal carbon dioxide partial pressure (PETCO2), was affected by wearing an N95 mask.
Careful peril-profit analyses of the potential long-term effects of masks are becoming increasingly important for patients and healthcare professionals. Although studies have been done on pregnant healthcare workers, the data on all healthcare workers during the coronavirus pandemic are unavailable.
2. Objectives
This study aimed to provide an initial, rapid, and scientific presentation of the risks and potential adverse medical effects of masks, particularly among specific diagnostic groups, patients, and other user groups.
3. Methods
The present case-control study was conducted over 3 months (July 1, 2020, to October 1, 2020) at Masih Daneshvari Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran. We examined 20 healthy medical personnel working in operating rooms (age 35 ± 8.3 years), including 66% males and 33% females. They were included in the study if they were under 65 years old and willing to follow the study protocol. Exclusion criteria were cerebrovascular diseases, convulsions, respiratory disease (pulmonary or inflammatory), ischemic heart disease, and uncontrolled blood hypertension or diabetes. We excluded workers with a baseline O2 saturation of less than 94% while breathing room air and who could not continue wearing the mask during the study. The study procedures were explained to all participants before the experiment started, and all participants were required to sign voluntary written forms, as mandated by the Declaration of Masih Daneshvari Hospital.
3.1. Procedure
Capnography, pulse oximeter, and cerebral oximetry were used to measure and record the participants' relative levels of excreted carbon dioxide (CO2), oxygen saturation (SaO2) of arterial pulsation, and rSO2. Their physiological parameters, including right-sided and left-sided rSO2, PETCO2, SaO2, pulse rate (PR), respiratory rate (RR), and mean arterial pressure (MAP) were measured before (time zero, baseline) and after they wore N95 masks during their routine working day in an operating room. As mentioned above, all physiological parameters were measured again after one and 3 hours of continuous mask-wearing. Correct connections and leak tightness were confirmed during the experiment. The present study defined physiological parameters as a negative effect of masks as a relevant measure.
3.2. Statistical Analysis
We used SPSS v. 21.0 (IBM, Armonk, NY, United States) to analyze the data. Mean ± standard deviations are provided. Statistical comparisons of the two groups were made using a two-tailed t-test (if the data was normally distributed) and the Mann-Whitney Wilcoxon test (if the data was not normally distributed). Statistical analyses were performed with Microsoft Excel 2010® (Microsoft, Redmond, Washington, USA). Differences were considered as 'not significant' with P values > 0.1, 'tendentially significant' (significant*) with P values between 0.1 and 0.05, 'significant' (significant**) with P values between 0.05 and 0.005, and 'highly significant' (significant***) with P values < 0.005. Correlations were defined as "negligible" with r values of 0.00 to 0.30 (-0.00 to -0.30), "low" with r values of 0.30 to 0.50 (-0.30 to -0.50), moderate" with r values of 0.50 to 0.70 (-0.50 to -0.70), and "high" with r values of 0.70 to 1.00 (-0.70 to -1.00). Figures were created with GraphPad Prism7© (GraphPad Software, California, USA).
4. Results
Results are given at three distinct time points for all 20 healthy volunteers. Physiological parameters of PETCO2, SaO2, cerebral oxygenic right-sided and left-sided (rSO2), PR, and RR were measured three times before (baseline) and after (60 min and 180 min) wearing N95 facemasks.
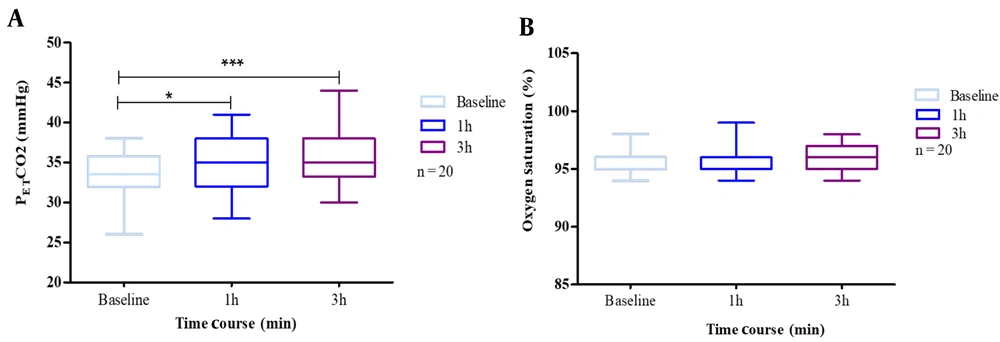
4.1. PETCO2 and SaO2
The results showed a significant change in PETCO2 concentrations, though this effect was not seen with SaO2. When participants wore masks, we found a combined effect of N95 respiratory protection and carbon dioxide rise. Nevertheless, we did not find a similar result for the decrease in oxygen saturation.
In addition, we addressed whether CO2 is increased due to mask-wearing. According to data, mask wearers showed outstanding frequency changes due to mask-wearing.
Mean PETCO2 at baseline was 32 (26.0 - 36.0) mmHg for non-mask-wearers, which rose to 35 (28.0 - 41.0) mmHg after 1 h and 38 (30.0 - 44.0) mmHg after 3 h of wearing an N95 mask. Therefore, we had a statistically significant increase (P < 0.05) in blood CO2 after continuous mask-wearing.
Baseline SaO2 was 96.0% (95.0% - 98.0%). The effect of masks was not statistically significant regarding blood oxygen saturation (SpO2) after the first, second, and third hours. The analysis results of SaO2 and PETCO2 are provided in Figure 1.
A, end-tidal carbon dioxide partial pressure (PETCO2); B, oxygen saturation (SaO2), at baseline, 1 h after, 2.5 h after. The exercise test was performed by 20 subjects with N95 masks. Box plots represent the changes in the oxygen saturation and carbon dioxide due to mask use. The results are presented as cases that used masks. The whiskers represent the local maximum and minimum values; each box's horizontal line represents the median. Differences were considered 'tendentially significant' (*) with P-values of 0.1 - 0.05 and 'highly significant' (***) with P-values < 0.005.
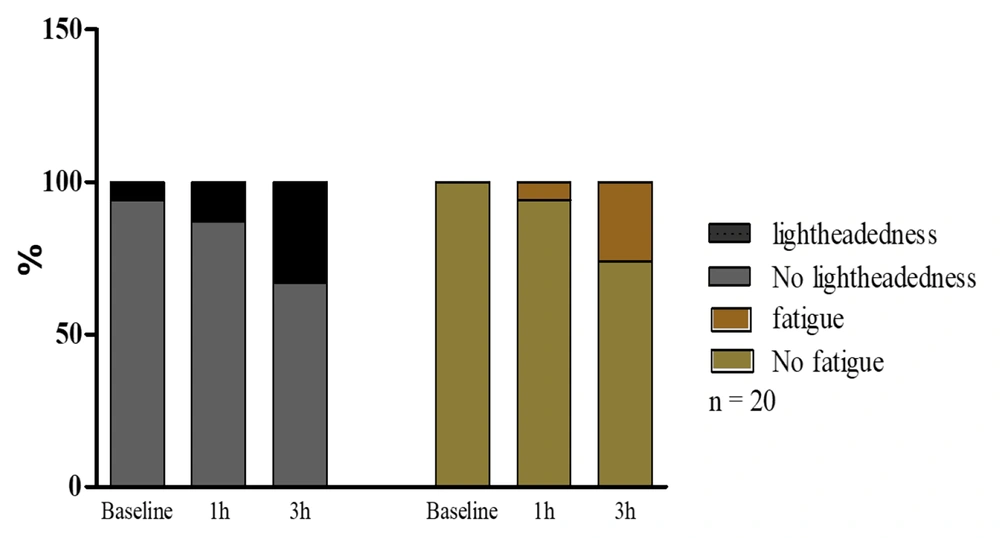
4.2. Lightheadedness and Fatigue
The study revealed that 25% (5 out of 20) of volunteers experienced lightheadedness. Furthermore, oxygen deprivation due to N95 respiratory masks was associated with fatigue in 20% (4 out of 20) of cases after 2.5 h of mask-wearing compared to the baseline. The dual occurrence of the physical parameters of lightheadedness and fatigue was found in 20% of the participants (Figure 2).
The occurrence of lightheadedness and fatigue before wearing a mask (baseline) and after 1 h and 3 h of wearing a mask. Lightheadedness and fatigue were increased after 1 h and particularly after 3 h. Nevertheless, it was insignificant in healthcare workers compared to the baseline after mask-wearing.
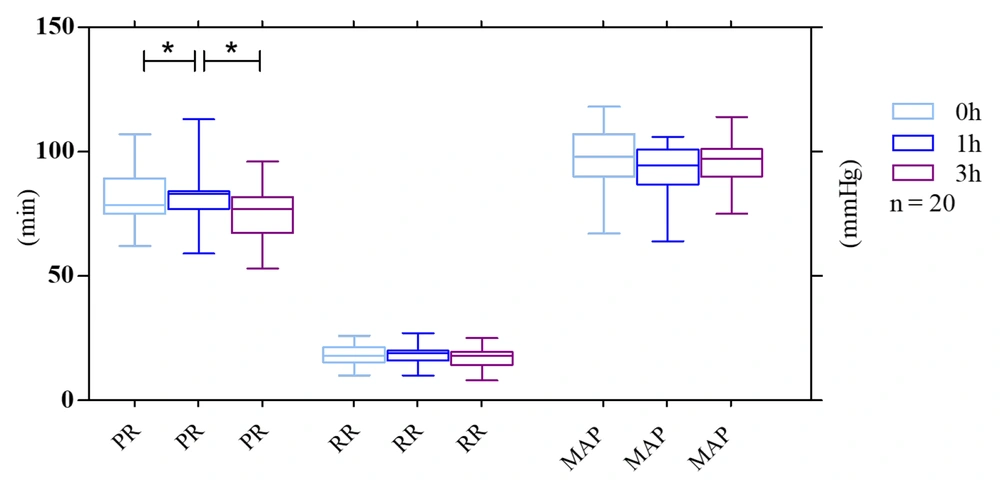
4.3. PR, RR, and MAP
Our results indicated that N95 facemasks can induce different effects on pulse rate (heart rate) at distinct time points. However, baseline PR while not wearing a mask was 82.0 (62.0 - 107.0) bpm vs. 83.0 (74.0 - 113.0) bpm after 1 h and 75.0 (57.0 - 96.0) bpm after 3 h, which represent a predictable increase in RR 1 h after mask-wearing. On the other hand, PR decreased to an amount lower than the baseline after a longer duration of mask-wearing.
Furthermore, the baseline RR was 18 (10.0 - 26.0) rpm vs. 18.0 (10.0 - 27.0) rpm after 1 h and 17.0 (8.0 - 25.0) rpm after 3 h. The results showed a decrease in RR, but not significantly after 3 h compared to the baseline.
Concerning MAP, the mean baseline was 96.0 (67.0 - 118.0) mmHg vs. 93.0 (78.0 - 106.0) mmHg after 1 h and 96.0 (75.0 - 114.0) mmHg after 3 h. After using masks for 1 h, we observed a reduction in blood pressure that was not statistically significant. It did not continue for the next 2 h and returned to the normal baseline measurement (Figure 3).
Mean changes in physiological parameters throughout the exercise test were performed by 20 subjects with surgical masks and N95 respirators. Pulse rate (PR, pulsations/min), respiratory rate (RR, breaths/min), and mean arterial pressure (MAP) at the three-time points were considered. The whiskers represent the local maximum and minimum values; each box's horizontal line represents the median. Differences were considered 'tendentially significant' (*) with P-values of 0.1 - 0.05.
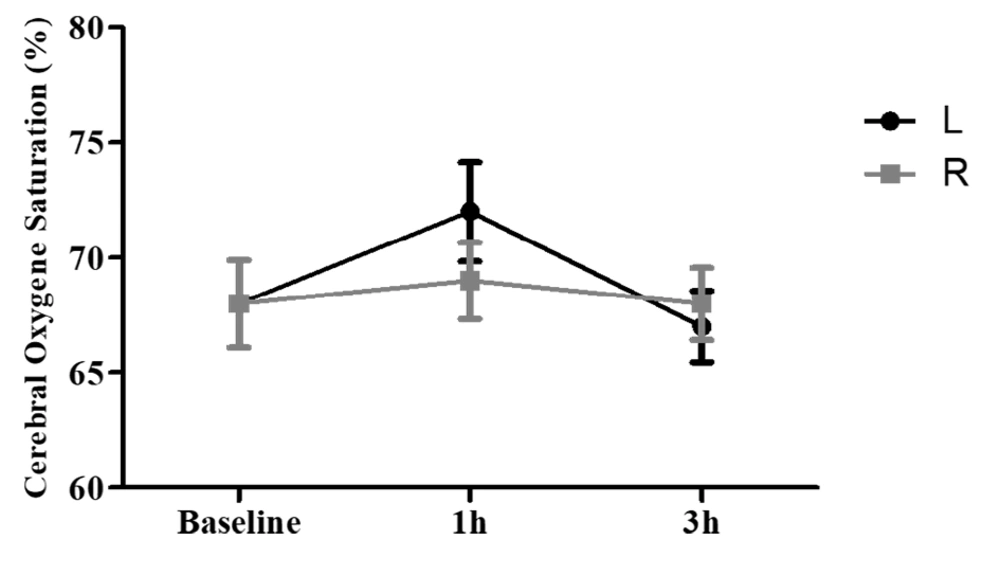
4.4. Cerebral Regional Oxygen Saturation
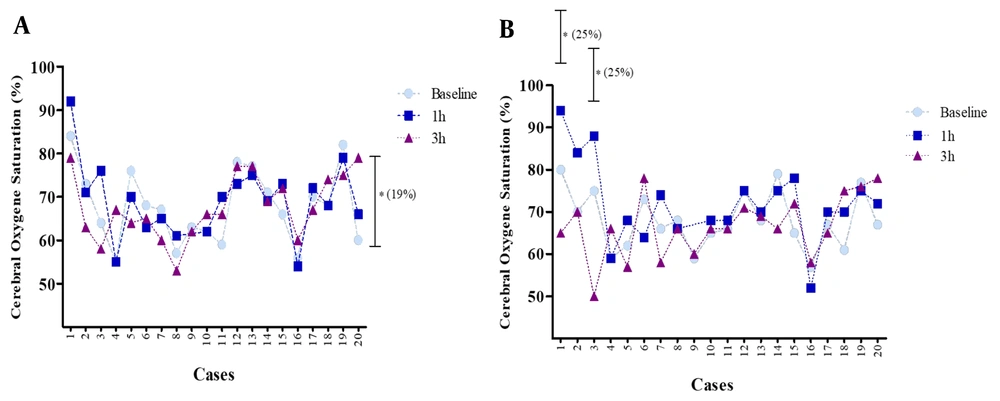
The mean rSO2 for the right and left parts of the brain following 3 h of using a mask showed no significant change (more than 20%) compared to the baseline (0 h) (Figure 4). The mean rSO2 right side at baseline was 68% (55.0 - 82.0), which rose to 69% (55.0 - 92.0) after 1 h and decreased to 68% (53.0 - 79.0) after 3 h of mask-wearing. Regarding the left side of rSO2, the mean value at baseline was 68% (59.0 - 80.0), which rose to 72% (52.0 - 94.0) after 1 h and decreased to 67% (50.0 - 78.0) after 3 h of mask-wearing. An increase in rSO2 (less than 20%) on the right side after 3 h compared with the baseline (0 h) was evident for some other individuals. Measurement of rSO2 on the right side for a volunteer at baseline was 84%, which rose to 92% after 1 h and decreased to 79% after 3 h of mask-wearing. Further, a few subjects who experienced a remarkable change showed a decrease in rSO2 (more than 20%) in the left side of the brain compared to the baseline.
The measurements of left-side rSO2 for three volunteers at baseline were 80%, 75%, and 66%, respectively, which rose to 94%, 88%, and 74% after 1 h. Furthermore, these values decreased to 65%, 50%, and 58% after 3 h of mask-wearing (Figure 5).
Relative changes in cerebral regional oxygen saturation (rSO2) on the right (A), and left (B) sides after using a mask compared to baseline (0 h) for each subject. Data are expressed as mean ± S.E.M. for each of the 20 cases * significant differences (more than 20% from baseline). Each dot indicates the oxyhemoglobin saturation in first‐degree and second‐degree venules at a specific point in time.
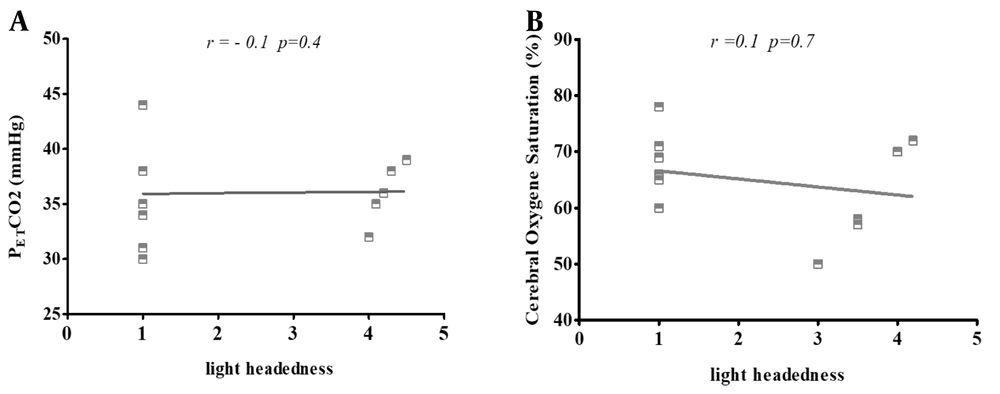
4.5. Correlation of Lightheadedness with PETCO2 and rSO2
We observed two statistically significant changes in physiological parameters during the study, both in PETCO2 and rSO2. Therefore, we did not have a statistically significant correlation in the quantitative analysis between PETCO2 and rSO2 and headache/fatigue as some accompanying factors among mask wearers (Figure 6).
Pearson's correlation scatters plot of lightheadedness with (A) end-tidal carbon dioxide partial pressure (PETCO2); and (B) cerebral oxygen saturation (rSaO2) in 20 subjects with N95 masks. The results present cases in which the use of masks exhibited no significant correlation with lightheadedness and two other variants. The correlation was defined as "negligible" with r values of 0.00 to 0.30 (-0.00 to -0.30), "low" with r values of 0.30 to 0.50 (-0.30 to -0.50), "moderate" with r values of 0.50 to 0.70 (-0.50 to -0.70), and "high" with r values of 0.70 to 1.00 (-0.70 to -1.00).
5. Discussion
The permanent use of N95s can influence cerebral oxygen, pulse rate, respiratory rate, and oxygen saturation, among others. The primary argument of this study is that masks influence the oxygen level of the brain and carbon dioxide levels. In contrast, blood oxygen saturation does not show any change. Previous studies have shown that N95 masks reduce the oxygen concentration of blood by more than 20 - 25%. Therefore, it is vital to thoroughly assess the risk factors of wearing facemasks for long durations (15, 16). It is also vital to have further studies to find an alternative for surgeons who frequently have long procedures in operation rooms. Wearing a mask can be associated with certain physiological parameters. However, no significant differences were found for all parameters (16-18).
Our results show that the use of a mask by healthcare workers does not have large and statistically consistent effects on key physiological parameters, such as oxygen saturation and respiratory rate, but does indeed affect pulse rate, carbon dioxide partial pressure, and cerebral oxygen. In the present study, health workers in operating rooms were impressed while wearing N95 masks at different time points. In addition, we observed increases in PETCO2 associated with mask-wearing. A small but significant increase in PETCO2 showed a clearer effect in our study, possibly due to CO2 rebreathing among the staff wearing masks. As in other studies, there were a few subjects with lung or heart diseases connected to PETCO2, which should be considered when wearing masks (19).
An increase in PETCO2 was also found with face masks used routinely (20). In another study, using N95 masks increased carbon dioxide tension/partial pressure (PCO2) among lung-healthy users but without a major physiologic burden (20, 21).
Trouble breathing with masks can be associated with certain neurological responses, such as increased impulses from the highly thermosensitive area of the face covered by the mask or temperature of the circulating air and an increase in PETCO2 (22). Our findings demonstrate that using a mask during aerobic training has minimal and statistically inconsistent effects on major physiological parameters such as HR, RR, BP, and SO2. Moreover, cerebral oximetry was reduced by 5 - 20% in our volunteers wearing masks. Values of rSO2 are affected by other physiologic variables that determine brain oxygen supply and demand. Alterations in most of these variables result in a symmetrical effect on rSO2 (13).
In order to avoid hypoxia in the brain, a sufficient source of oxygen is critical. The amount of rSO2 levels is sensitive to changes in both arterial oxygen and carbon dioxide blood content (23). Maintaining adequate oxygen delivery to tissues and organs, particularly the brain, is of fundamental importance. The dangers of prolonged hypoxia and reduced oxygen delivery to the brain are well documented (24, 25).
The parameters of right-sided and left-sided rSO2 showed different patterns. In the first hour of mask-wearing, we saw an increase in right and left-side rSO2 of the brain; however, the increase in rSO2 on the left side was higher compared to the baseline, which was more than the amount of rSO2 on the right side of the brain. Normally, differences of up to 10% in cerebral oxygenation between the left and right hemispheres are apparent, especially during unstable arterial saturations, which may indicate uneven cerebral oxygenation (1).
Increases in PETCO2 after 1 h can be associated with increased cerebral oxygen in the brain's left and right sides. One reason may be that cerebrovascular response to the hypoxic condition is caused by increased PETCO2 after mask-wearing. Therefore, PETCO2 can be used as the stimulus for increased cerebral oxygen. The increase was insignificant; after 3 h, cerebral oxygen decreased to values even less than the bassline (26). The effects of oxygen deficiency overcome vasodilation due to increased carbon dioxide, and we observed a decrease in rSO2 after a 3 h exposure to the hypoxic situation (mask). Another reason could be more oxygen consumption over time, followed by more mental work than the baseline, which can cause a decrease in rSO2. Earlier studies demonstrated that rSO2 is affected by changes in cardiac output and oxygen consumption. However, it seems to be independent of MAP (as cerebral oxygen saturation), which changes in arterial oxygen content (i.e., hypoxemia, anemia) and could have an impact on cardiac outcomes and corresponding rSO2 (27).
It is known that cerebral metabolic rate is related to oxygen delivery. Due to decreased arterial oxygen, we will have decreased oxygen delivery. In this case, this cerebral autoregulation mechanism maintains cerebral oxygen delivery via proportionate increases in cerebral blood flow.
In the case of steady arterial oxygen content, decreases in rSO2 imitate an increased oxygen extraction ratio. A reduction in cerebral oximetry values > 20% from baseline has been identified as a reliable, sensitive, and specific threshold for detecting brain ischemia (28).
In previous studies focusing on healthy people, surgical face masks were associated with an increased heart rate (29). Our results demonstrate that wearing masks in two ways can influence pulse rate. After just one hour of mask-wearing, we saw an increase in pulse rate. We observed a decrease compared to the baseline after three hours of mask-wearing. An increase in PR might result from an O2 decrease in tissue in the form of demand of the body to compensate for reducing oxygen via cardiac output, causing an increase in PR. As the body wants to compensate for the decrease of oxygen via cardiac output, PR will increase (30). Increased activity of breathing or respiratory muscle to increase cardiac output can cause an increase in PR (2, 29).
Furthermore, as inducing cardiac output cause of hypoxia condition is restricted in both diastolic and systolic diameters, we had a decrease in PR after 3 h of mask-wearing (31). On the other hand, vasodilation could cause an increase in arterial blood flow and help decrease arterial heart rate and blood pressure (27).
Concerning MAP, we saw an increase after one hour of mask-wearing and a decrease after three hours. Normally, based on a previous study, the pulse rate increase is associated with higher blood pressure. This could have the same template and explanation for MAP with PR (32).
One possible explanation for participants who experienced headaches can be the compression of sensitive facial skin and superficial nerves by the face mask and its tight straps, especially when worn longer (33).
Based on the previous study, rSO2 and PETCO2 can contribute to fatigue and lightheadedness. The correlation of lightheadedness with PETCO2 and rSO2 in the present study was insignificant, although a correlation existed. This may be due to the number of studied cases, which were insufficient for correlation analysis (33, 34).
There were a few limitations in the present study that may complicate our results:
1. Certain brain areas remained unmonitored as cerebral oximeters only measure regional cerebral oxygen supply.
2. The effect of N95 masks was tested on only a few persons, which could be a reason for significant differences after wearing the mask compared to the baseline value. Other factors like BMI should also be addressed. Moreover, the physiological effect of mask-wearing can vary in different situations with different physical actions.
3. The duration of mask-wearing should be extended and not restricted to just three points in time. Further time points would provide more insight into the effect of mask-wearing on cerebral oxygen levels or oxygen saturation.
5.1. Conclusions
The purpose of wearing facemasks is to protect the wearers against viruses. It is important to determine whether masks can provide sufficient protection for healthcare workers while affecting various physiological parameters, particularly cerebral oxygen saturation, carbon dioxide partial pressure, PR, and MAP.