1. Background
Major surgeries particularly CABG under CPB can trigger inflammatory reaction which has been compared with systemic inflammation response syndrome (SIRS). The induced stress response causes release of cortisol, catecholamines and other stress hormones which leads to insulin resistance and hyperglycemia. A strong association has been demonstrated between this hyperglycemia and adverse clinical outcomes. The increased serum glucose level can lead to monocyte and neutrophils functions impairment and increase the generation of superoxide radical and pro-inflammatory cytokines (1-3). Cardiac arrest, aortic cannulation, iatrogenic trauma, transfusion, blood component exposed to non-physiologic surfaces, temperature fluctuations, and ischemic reperfusion injury can induce a profound stress response which is associated with the release of cytokine and reactive oxygen species (ROS) generation, leading to cardiac tissue damage (4-8). Despite the significant progression in anesthetic methods and surgery strategies, stress response still remains as a challenging unsolved problem with several adverse clinical outcomes. Considering the importance of the issue, doing research to find new effective interventions with more cardio protective effects seems necessary. Optimistically, any strategy with antioxidant properties might limit adverse outcomes of this SIRS-like reaction. According the results of previous clinical studies, Selenium (Se) could be an acceptable candidate for cardio protective goals (4, 9, 10). There is good evidence that Se has potentially cardio protective properties, which could modulate several injurious mechanisms of inflammation and provide significant effects on the course and clinical outcomes of stress inducted inflammation (11). Clinical evidence established that Se serum levels drop during CABG. On the other hand, post injury normalization of Se status is unable to reduce oxidative stress (2, 12-14). Hence, a preemptive approach was chosen. It is noticeable that the optimal dosage and method of administration, selecting cases, and many other questions are not answered yet. Moreover, due to the adverse impact of elevated levels of serum Blood Sugar in surgery and valid literature indicating a positive correlation between stress response and BS, we selected blood sugar as stress response marker.
2. Objectives
The purpose of this work was to provide proof of concept that Se treatment can reduce stress response and inflammation in patients undergoing CABG surgery.
3. Methods
3.1. Setting
This prospective single-center randomized clinical trial was conducted at Dr. Heshmat hospital. This governmental/public center is affiliated to Guilan University of Medical Sciences (GUMS), Rasht, Iran. It is a specialized and referral hospital with one hundred and eighty beds provided for different types of cardiac surgeries. The trial protocol was approved by the research ethics committee of GUMS and the trial was registered in the Iranian registry of clinical trial (IRCT) with the code 2015040813456N3.
3.2. Study Participants
First, an informed consent was taken from all participants. Then, their information was collected, including demographics, medical record data, preoperative risk factors, and medications.
3.3. Inclusion Criteria
Patients who were candidate for elective CABG under CPB as an isolated procedure, aged 30 - 65 years, had 3 vessel diseases, and ejection fraction > 40% - 45% were selected for the study.
3.4. Exclusion Criteria
The patients were excluded from the study if there were any condition including emergency surgery, myocardial infarction within the last 6 months, supplemented by antioxidants during the previous month, concomitant malignant disease, diabetes, thyroid disease, liver or renal dysfunction, pregnancy, major trauma or major surgery during less than three months before the current surgery, skin cancer, and autoimmune disorders.
Sample size: It was calculated that a minimum sample of 36 patients in each group was required based on a margin of error α = 0.05 and β = 10%, expected power of 90%, and Z value of 1.28. However, we decided to enroll 40 patients in each group.
3.5. Randomization and Blinding
Seventy three consecutive patients were randomly enrolled in either Se group (S) or control group (C) using randomized fixed quadripartite blocks. Our subjects had an equal possibility of being assigned to each of the two groups. Se and normal saline were injected intravenously as a single bolus dose by an aware anesthesiologist who was ready to face any adverse event. However, the patient and investigator were blinded.
3.6. Intervention
Patients in the group S received an intravenous bolus of 600 μg Se in the form of sodium-selenite (Biosyn Arzneimittel GmbH. Fellbach, Germany. vial 50micrograms/ml), diluted with 20cc sodium chloride 0.9% within 30 minutes, while the group C received normal saline, both prior to surgery. To ensure physical and mental health, a medical history and physical examination were carried out. All samples were taken in the morning between 7:30 am to 8:30 am for baseline biochemical serum measurements as well as blood sugar.
3.7. Anesthesia and Surgical Methods
Anesthesia was performed on the two groups in accordance with the hospital standardized protocol for CABG surgery (15). Surgery always started in the morning between 8: 00 am to 9: 00 am to avoid bias caused by the circadian rhythm of circulating stress hormones. Our subjects in both groups received oral lorazepam 1mg the night before surgery, one hour before transferring to the operating room, and intramuscular morphine 0.1 mg/kg half an hour before transferring to the operating room as premedication. On arrival in the operating room, an intravenous catheter 18 gauge was inserted into the forearm vein. All patients underwent standard monitoring including electrocardiography, pulse oximetry, non-invasive blood pressure (NIBP), arterial catheter, central venous pressure, nasopharyngeal thermometer, Bispectral index(BIS), and End tidal Co2 ( Etco2). General anesthesia was induced with etomidate 0.3 mg/kg, sufentanyl 0.3 µg/kg, and cisatracorium 0.2 mg/kg. A low dose of etomidate 0.03 mg/kg was administrated to blunt etomidate-related myoclonus (16). Anesthesia was maintained with continuous infusion of propofol 50 - 150 mg/kg/min, sufentanil 0.1 - 0.3 µg/kg/h, and cisatracurium 0.6 mg/kg/h according to patient’s hemodynamic statue. Within the controlled mechanical ventilation, respiratory rate and tidal volume were adjusted to keep Etco2 of 35 - 45 mmHg. The timing and type of any treatment within the surgery were based on the patients’ condition.
To achieve an activated clotting time (ACT) above 480 sec, an initial dose of 300 u/kg of heparin was administrated and then, CPB was performed. The patients underwent median sternotomy and a standard technique was applied to establish heart-lung pump (standard membrane oxygenator Medtronic). At the end of the vascular graft, protamine was used in a ratio of 1:1 to fully reverse the heparin effect. Also, at the end of the surgery in stable vital signs, the patients were transferred to coronary care unit (CCU). When standard criteria including stable vital signs, being awake and cooperative, normal sinus rate, normal electrolytes Na/K/Mg, arterial blood gas (ABG), and body temperature of 36 - 37°C were achieved, weaning process and tracheal extubation were performed within 6 - 8 hours. Our cases were monitored closely for any side-effect due to the intervention. If any clinical adverse effect was observed, Se concentration was immediately measured, symptomatic therapy started, and the patient was excluded.
3.8. Blood Testing
Blood samples were taken at four point times including before induction of anesthesia (T0), at the end of CPB (T1), 24 hours (T2) and 48 hours (T3) after surgery. All analyses were performed in the hospital laboratory. 5 ml of blood was sampled by using a heparinized syringe and immediately placed in a cold box and sent to the laboratory where BS was measured by photometric analysis and glucose oxidase. A specific quantitative diagnostic kit was used for this purpose.
3.9. Statistical Analysis
All statistical analyses were performed using SPSS statistical software version 16 (SPSS Inc, Chicago, II). Chi-square test, K-S test, and repeated measurement tests were applied for statistical analysis. Continuous and categorical variables were presented as mean standard deviation or percentages. All reported P-values below 0.05 were considered statistically significant.
4. Results
The results of the present study showed that there were no significant differences between the two groups regarding baseline demographic and clinical data (Table 1). The procedural characteristics were also similar in both groups. In the present study, 80 subjects were divided into Se (S) and control (C) groups. In group (S), two patients could not be extubated within the expected time and instead, intra-aortic balloon pump was used. In group (C), two patients needed valve repair during surgery and one was affected by malignant hyperthermia. By excluding these cases from the survey, the final data obtained from 73 patients were analyzed.
| Variables | Control Group (n = 37) | Selenium Group (n = 36) | P Value |
|---|---|---|---|
| Age, year | 57.46 ± 8.63 | 54.94 ± 8.57 | t = 1.18, P = 0.24 |
| Male (%) / Female (%) | 27 (72.97%) / 10 (27.02%) | 25 (69.44%) / 11 (30.55%) | P = 0.33 |
| BMI, Kg/m2 | 27.12 ± 2.37 | 27.07 ± 3.03 | t = 0.035, P = 0.97 |
| Ejection fraction (%) | 47.63 ± 7.21 | 48 ± 3.51 | t = 0.25, P = 0.79 |
| Surgery duration, hours | 2.86 ± 0.36 | 2.81 ± 0.28 | t = 0.68, P = 0.49 |
| Pump time, minutes | 61.32 ± 18.28 | 58.05 ± 13.01 | t = 0.81, P = 0.41 |
| Clamp time, minutes | 37.63 ± 10.11 | 35.19 ± 8.87 | t = 1.04, P = 0.31 |
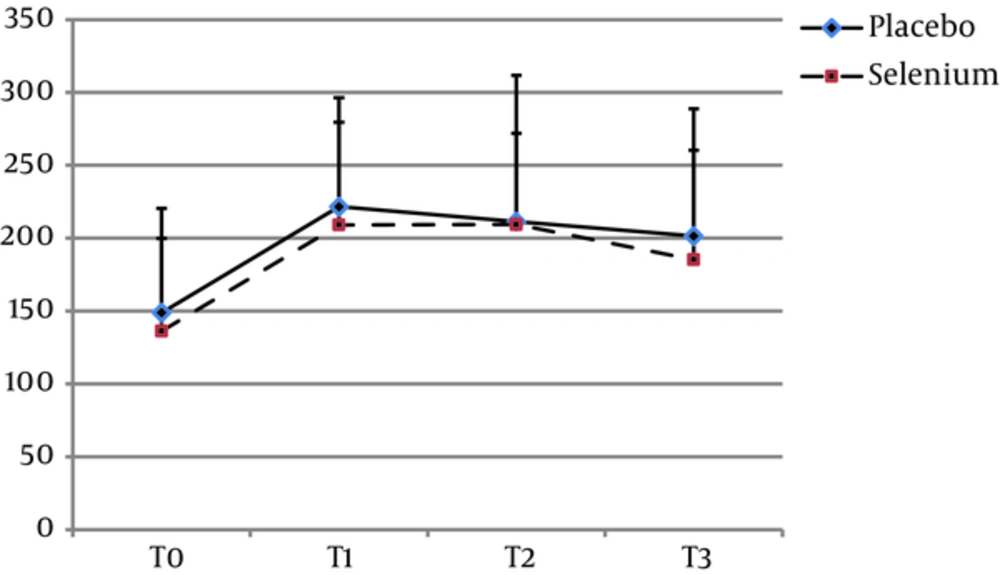
The mean age of the patients in groups S and C were 54.94 ± 8.57 and 57.46 ± 8.63 years, respectively (P = 0.24). In group S, 25 (69.44%) and in group C 27 (72.97%) of participants were male (P = 0.33). There was no significant difference between the two groups regarding the other baseline characteristics including BMI (Kg/m2) (P = 0.97), ejection fraction (P = 0.79), surgery duration (P = 0.49), pump time (P = 0.41), and clamp time (P = 0.31) (Table 1). In both groups, a sharp rise in BS levels were observed following CPB (P = 0.0001). Although the trend of BS changes was significant in both groups (P = 0.0001), there was no statistically significant difference between the groups at all point times T0 (P = 0.45), T1 (P = 0.48), T2 (P = 0.92), and T3 (P = 0.42). None of the patients in selenium group experienced adverse effects due to the intervention.
| Variable | Groups | T0 | T1 | T2 | T3 | P Value | P Value |
|---|---|---|---|---|---|---|---|
| Blood Sugar | Placebo | 148.8 ± 71.6 | 221.6 ± 74.8 | 211.4 ± 100.4 | 201.5 ± 87.4 | P = 0.001 | P = 0.837 |
| Selenium | 136.2 ± 63.6 | 209.1 ± 70.5 | 209.4 ± 62.5 | 185.3 ±75.1 | P = 0.001 | F = 0.215 | |
| P value | P = 0.45 | P = 0.48 | P = 0.92 | P = 0.42 | |||
Abbreviations: T0, before induction of anesthesia; T1, at the end of CPB; T2, on the 1st day after operation; T3, on the 2nd day after operation.
5. Discussion
The stress response which is mainly mediated by sympathoadrenal system and hypothalamic -pituitary- adrenal pathways causes neuroendocrine reactions described by extreme gluconeogenesis, glocogenolysis, and insulin resistance. The inflammatory response to surgery leads to additional hepatic output of glucose, pro inflammatory cytokines and sympathoadrenal system act together to induce high BS. Moreover, inflammatory mediators such as TNF-α, IL-6, IL- 1, and C-reactive protein also result in peripheral insulin resistance (3, 17).
Studies have documented that intraoperative elevated glucose during CPB and hyperglycemias is associated with increased risk of major complications including prolonged ventilator requirements, increased hospital stay stroke, renal failure, atrial fibrillation (AF), infection and mortality regardless of the preoperative diagnosis of of DM (18). Tatsishi W et al. in 2014 (19) found a positive correlation between post CABG maximal BS and AF. Responsible mechanisms have been discussed in previous studies, which declare that increased serum glucose concentrations can affect cardiac electrical functions, Ca2+ handing impairment, and prolonged p-wave dispersion (20, 21). According to the abovementioned statements indicating the significance of BS, it was chosen as the marker of stress response and inflammation in our study. All factors that could affect blood sugar levels were as much as possible the same in the two groups. These factors included underlying diseases, patients’ medications, level of anesthesia according to BIS, and pain control by administration of morphine 0.1 mg/kg and apotel 1gr per 6 hours. Also, all surgeries were performed by one surgical team. Recently, the cardio protective effects of various strategies have been tested to blunt the stress response due to surgery. In this regard, trace elements due to their promising results have attracted lots of attention. Selenium as a unique trace element is essential for various aspects of human health and presents its beneficial effects through at least 25 certain selenoproteins (SePs). Among them, selective SePs such as selenoprotein P has been used as stress response marker (22-25). On the other hand, it has been proven that during CABG surgery, Se serum levels decrease in correlation with high CRP values, which translate to poor outcomes. Low Se concentrations have also been observed in the inflammatory state, which is represented with reactive oxygen species generation by activated macrophages, causing oxidative stress and tissue damage (11). Indeed, Se prevents ROS production and neutrophil adhesion to endothelial cells; accordingly, it plays an important role in the body’s defense against oxidative stress. In several studies, Selenium as supplement has been used in a wide range of dosage with no documented adverse effects (2, 13, 26). However, not completely in line with these findings, there are clinical studies reporting warning results, which are partly noted here. It is notable that some studies have documented that high serum levels of Se may induce a pro-oxidant impact (11). Harmful effects such as carcinogenesis, cytotoxicity, and genotoxicity are supposed for this trace element. Synthesis of thyroid hormones, growth hormone, and insulin-like growth factor-1 may also be affected. Moreover, hepatotoxicity, gastrointestinal disorders, hair loss, brittle nails, garlic smell in exhaled air, nausea and vomiting, dizziness, and pulmonary edema have been reported (1, 24, 25, 27-30). In general, it seems that the current knowledge about pharmacodynamics and safety of Se is incomplete; hence, routine supplementation in not exactly selected cases must be avoided. Previous studies have indicated that to achieve beneficial effects, Se should be administrated at dosage above 500 μg (2, 12). A concern about our high risk patients led us to choose the lowest dosage in the effective range which was chosen as 600 μg. In both groups, BS markedly increased during CPB, which points to its involvement in inflammation process and stress reactions. The highest level was observed at the end of CPB, which gradually decreased at the next measurement point times. In this manner of intervention, no statistically significant difference was found between the two groups at all time points, which rejected the authors’ hypothesis. However, several advantages of Se such as simple administration, cost effectiveness, and minimum side-effects in short-term use, in addition to its confirmed antioxidant properties, may encourage us for further studies. Indeed, our high risk studied group and lack of enough related trials prevented us to test higher doses with earlier and longer treatment duration to achieve the beneficial effects. Nevertheless, we hope our achievements in this research indicating the safety of intervention with Se in this patient population would be considered as a basis for studies in this field. We point out in the following to a number of clinical studies which have focused on the cardio protective effects of Se. There are some studies dealing with the effects of Se under different conditions on patients other than pump CABG patients. Considering the limited number of clinical trials in this field and especially lack of any study exactly focusing this subject, the innovation of this work is highlighted. However, comparing our findings in this study with those of similar studies and discussing the conflicting results are restricted.
Leong J et al. (10) in 2010 examined the therapeutic effects of two-week Se administration (200 µg /day) in combination with coenzyme Q10, omega 3, lipid acid, and orthotic acid. They concluded that antioxidants would act more effectively if applied as a network. However, the exact role of Se was not inferred in this survey. Studies have made it clear that different types of cardiac surgery can induce different levels of stress response. Accordingly, combined procedures were not investigated in this survey. However, by comparing the two studies in terms of study units, a remarkable difference was observed. They had selected their patients among those requiring both CABG and valve surgeries; therefore, our cases were expected to experience a greater degree of stress response. Moreover, longer duration of Se treatment in combination with other antioxidant agents as well as lower degrees of stress responses can be effective factors to explain their better results. Altaei et al. in 2012 (4) found that Se supplementation (140 µg × 3 Cap per day), three days before CABG significantly suppressed cytokines release. It is noticeable that Se dosage, timing, and the rout of administration were different from those in our study. Additionally, their patients had been selected from both on-pump and off-pump CABG patients with different degrees of stress response severity.
Sedighinejad et al. in 2016 (9) conducted a study to determine whether administration of intravenous Se (600μg) before CABG could decrease the inflammatory response reflected by CRP, IL-6, and TNF-α. Their results demonstrated just a borderline significant superiority of this treatment based on CRP levels during the first hours of surgery. They supported the idea that long-term effects of Se seem to be limited. Stopp et al. in 2013 (2) performed a study on patients undergoing elective cardiac surgery. The patients received an intravenous bolus of 2000 µg Se after induction of anesthesia followed by 1000µg/day during the days of the intensive care unit stay. Se concentrations were measured at regular intervals. Se serum levels were reported normal which were associated with a decrease in SOFA scores at ICU admission time. Their findings also indicated that the positive effects of this trace element were not long-lasting and even high doses of Se could not prevent Se level drop within the next days. Several factors may explain the inconsistent findings of different studies. In fact, due to the genetic background of the individuals, inflammatory response varies significantly among the patients, and the main triggering factors in the onset of systemic stress response in cardiac surgeries are not well known (i.e. cardiopulmonary bypass, surgical trauma, etc.). Moreover, it is known that Se has a complex biology with several effects on the other metabolic pathways that is affected by the disease process itself. In addition, kinetics of exogenously administration of Se and its pathophysiological role in multi-organ failure is little understood (31). It is supposed that patients’ genotype and phenotype might affect Se distributions. It should also be noted to the role of varying doses of Se and preparation method, route of administration, anesthetic technique, surgeon’s experience, and selected anesthetic agent, as well (27, 32-35).
Limitations: The authors acknowledge the fact that this trial describes a single-center experience with a small sample size. In addition, the evaluated stress response marker was restricted to BS. In fact, it could not be ruled out that the results might be different if other indices were measured. Moreover, the mentioned point times might not have been optimal to detect the peak values. In spite of the mentioned limitations, these results yield a valid conclusion.
5.1. Conclusion
We could not find a meaningful difference between Se recipient and control groups. Therefore, our hypothesis was not proven. Thereby, higher doses with repeated, timely treatment may be needed to achieve beneficial effects. This study supports further well-planned trials, aiming to find the optimized dosage, root, and timing of Se administration.