1. Background
Intestinal edema can be observed intraoperatively in case of laparotomies, especially lengthy operations. It is more difficult to perform anastomoses, and there is a higher chance of anastomotic leakage when the gut walls are swollen (1).
There is evidence that the availability of oxygen and blood flow to the intestinal wall durin surgery and the initial postoperative period are other significant factors impacting the repair of bowel anastomoses. Intestinal edema reduces interstitial oxygen tension and intestinal blood flow, affecting microbial defense and healing. When performing intestinal anastomoses, it is evident that precautions should be taken to prevent the development of bowel edema (2).
Because even slight hypovolemia affects blood flow to the colon, fluid restriction is not advised during bowel operations. Therefore, to avoid decreased intestinal perfusion during laparotomy procedures, intravascular fluid deficits must be corrected. These deficits arise from preoperative dehydration, anesthesia-induced hypotension, bleeding, and evaporative water loss (3).
In patients with elective colon cancer mass excision and anastomosis, there is growing support in the literature for the use of targeted fluid therapy that is guided by dynamic markers of fluid responsiveness, which could decrease the incidence of intraoperative hypotension, dehydration, and perioperative complications (4).
The present study hypothesizes that pulse pressure variation (PPV) is a good minimally invasive tool that guides intraoperative fluid therapy for patients undergoing open resection of colon cancer mass and anastomosis to obtain the optimum volume of fluids that maintain hydration without hemodynamic instability and with less postoperative bowel edema, anastomotic leak, rapid bowel recovery, and shorter hospital stay.
2. Objectives
For colon cancer patients with open mass resection and anastomosis, this study aimed to compare the effects of intraoperative fluid therapy based on PPV to traditional fluid therapy to maintain adequate hydration without complications.
3. Methods
This prospective, double-blinded, randomized, controlled study was carried out at Eldemerdash Hospital in Cairo, Egypt, in accordance with the approval obtained from the Research Ethics Committee of Ain Shams University under IRB number 00006379. This study was registered at ClinicalTrials.gov (clinical trial ID: NCT05502835) and conducted in accordance with Consolidated Standards of Reporting Trials 2010. This study was conducted from September 2022 to January 2023. Prior to the intervention, informed consent was obtained from the patients.
The study enrolled 90 patients (males and females within the age range of 18 - 70 years) with physical status I and II, according to the American Society of Anesthesiologists (ASA), who were admitted for the elective open resection of colon cancer mass and anastomosis. The exclusion criteria included patient refusal, an ejection fraction of less than 30%, severe cardiac arrhythmia, peripheral artery disease, pulmonary pathology preventing using tidal volume greater than 6 ml/kg by mechanical ventilation, and the presence of hepatic and renal dysfunction.
Bowel preparation was performed according to the surgeon’s instructions the day before surgery. The patients fasted according to standard “nothing by mouth” guidelines. All the patients received premedication with 3 mg bromazepam tablets the night before and the morning of surgery. A peripheral intravenous line (18G) was installed in the operating room. After induction with propofol 2 mg/kg, fentanyl 2 μg/kg, and atracurium 0.5 mg/kg as a neuromuscular blocker, general anesthesia with endotracheal intubation was performed. Monitoring included pulse oximetry, noninvasive blood pressure, electrocardiography, capnography, anesthetic agent analyzer (to maintain an estimated 1.2 minimum alveolar concentration), and urine catheter. Moreover, anesthesia was maintained with isoflurane in combination with oxygen. All the patients received ventilation at the rate required to keep an end-tidal carbon dioxide value between 35 and 40 mmHg. The patient’s anesthetist administered analgesia and adjusted the patient’s anesthetic dosage.
The patients were randomized using random numbers generated by a computer and with the sealed envelope technique into one of the two following groups:
Group A (n = 45) (conventional fluid management [CFM] group)
Group B (n = 45) (PPV-based goal-guided fluid management [GGFM] group)
All the patients were operated in the supine position.
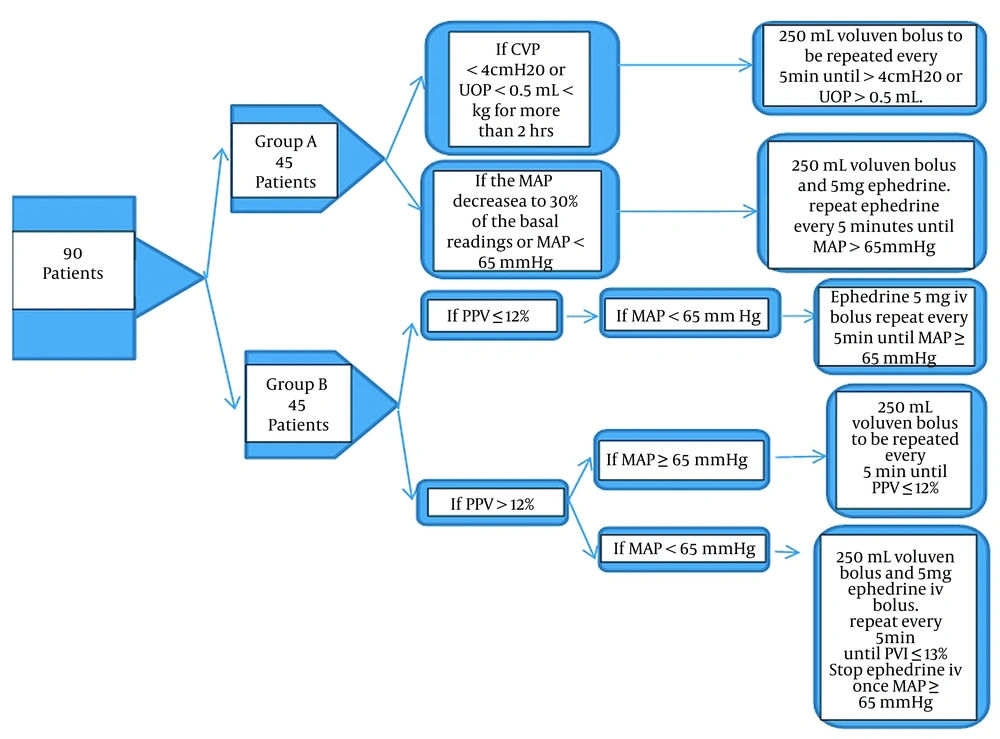
In group A, a 7 Fr. 20 cm long, triple-lumen central venous pressure (CVP) measuring catheter (Certofix®, B. Braun, Germany) was inserted into the patient’s right jugular vein via an ultrasound-guided approach. The patients received intravenous fluids in accordance with the standard surgical practice of 6 mL/kg/hour (from incision to skin closure). In order to be considered hypotensive, the patient’s mean arterial pressure (MAP) had to be at least 30% lower than baseline or less than 65 mmHg. In this case, a colloid infusion (Voluven® 6% Fresenius, Germany) was started, and 5 mg intravenous ephedrine was given. Every 5 minutes, ephedrine was administered again until the MAP reached 65 mmHg. The patients in group B were secured with a radial artery catheter (20G) to continuously monitor blood pressure and PPV after anesthesia induction. Baseline PPV values were noted, and then the patient was continuously monitored in addition to standard monitoring by the CARESCAPETM B650 monitor (General Electric Company, USA).
The patients in group B received intravenous fluids at 2 mL/kg/h. If PPV was greater than 12% for more than 5 minutes, a bolus of 250 mL colloid (6% Voluven®) was administered. If PPV was still greater than 12% after the fluid bolus infusion, it was repeated every 5 minutes until the PPV was less than 12%. An intravenous bolus of 5 mg ephedrine was administered as needed to maintain MAP above 65 mmHg during this procedure. In cases where PPV was < 12% and MAP < 65 mmHg, ephedrine 5 mg was administered intravenously and reapplied every 5 minutes to keep the MAP over 65 mmHg (Figure 1).
After the resection of the colon mass, a 3 × 3 cm specimen of the colon was obtained prior to the anastomosis. Each sample was weighed immediately after collection (wet weight). The samples were evaporated to constant weight (dry weight) in a drying cabinet at a temperature of 60°C for 24 hours. Then, (wet weight - dry weight)/dry weight was used to measure each sample’s water content (fraction). The water content (fraction) of each sample was then calculated (wet weight - dry weight/dry weight).
The treating anesthetist decided to administer additional boluses of crystalloids, colloids (hydroxyethyl starch), or blood products based on a subjective estimate of blood loss. All the patients were given heat and moisture exchangers, body warmers, and fluid warmers as part of the effort to prevent intraoperative hypothermia. If patients experienced postoperative pain, 0.5 mg/kg intravenous pethidine bolus was used as rescue analgesia to control pain.
Intraoperative fluid volume and hemodynamics are recorded after anesthesia induction and hourly until the end of surgery. In addition, length of hospital stay, initial gastrointestinal movement, postoperative anastomotic leakage, and bowel edema were recorded. The principal investigator performed a postoperative follow-up of each patient, and the data on the time to return of intestinal movement, intestinal leakage, and length of hospital stay were collected.
3.1. Study Endpoints
The primary endpoint was the amount of intraoperative fluid volume. The secondary endpoints were water fraction to detect bowel edema, intraoperative MAP, heart rate, urine output, blood loss, ephedrine use, postoperative days for bowel recovery, bowel leakage, and hospital stay.
3.2. Sample Size Calculation
According to a study conducted by Cesur et al. (5), power analysis predicted that each group would require 30 patients and that GGFM would lower the volume of crystalloids by 20%. The sample size was calculated using the sample size calculation PASS software (version 11.0). Group sample sizes of 30 achieved 95% power with a significance level (alpha) of 0.05. However, given the possibility of excluding patients, the number of patients in each group was set to 45.
3.3. Statistical Analysis
The chi-square test was used to ascertain the relationship between categorical variables. The Student’s t-test was used for parameters with parametric distribution between the two groups. The Mann-Whitney U test was used to test for non-parametric distribution between the two groups. As a result, the P-value was deemed significant, non-significant, and highly significant in the case of P < 0.05, P > 0.05, and P < 0.01, respectively.
4. Results
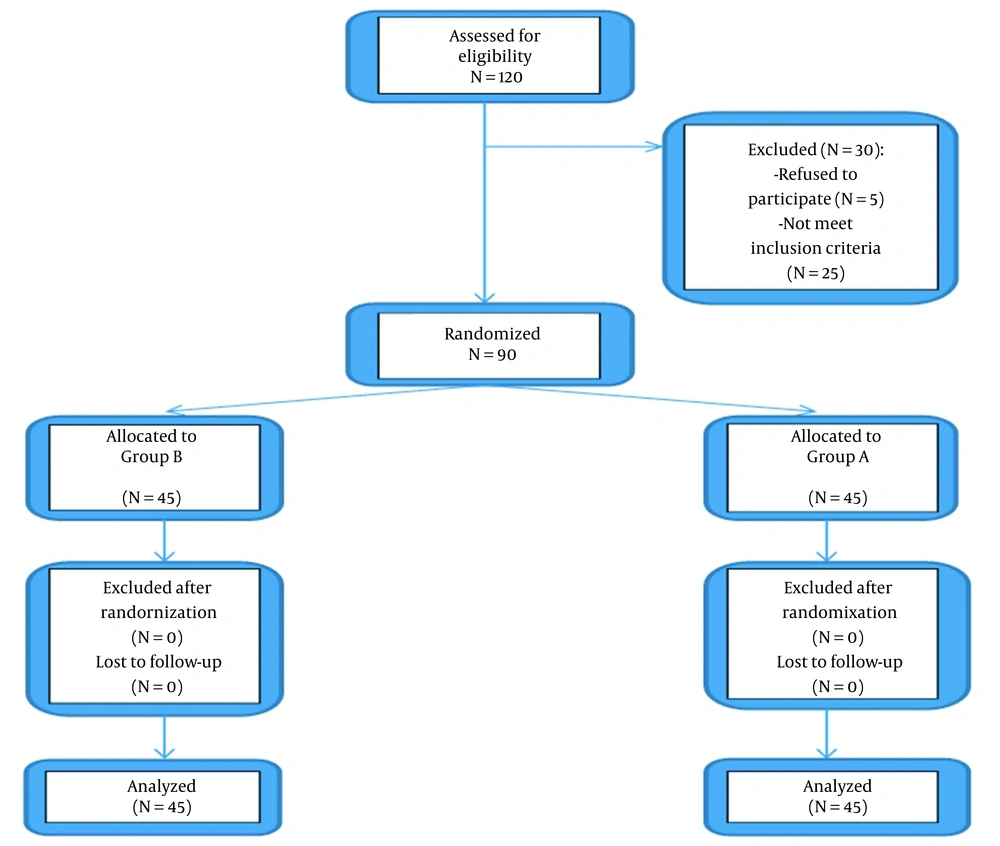
As illustrated in the CONSORT flow diagram, a total of 90 patients were enrolled and analyzed in the study (Figure 2).
4.1. Demographic Data
There was no significant statistical difference between the two groups in demographic data (P > 0.05), as shown in Table 1.
| Variables | Group A | Group B | P-Value |
|---|---|---|---|
| Age (y) | 55.8 ± 9.6 | 53.5 ± 12.1 | 0.32 |
| Gender male/female (No.) | 23/22 | 22/23 | 0.83/0.83 |
| Weight (kg) | 79.9 ± 11.3 | 77.1 ± 11.5 | 0.24 |
| Height (cm) | 158.8 ± 16.3 | 161.4 ± 5.3 | 0.31 |
| ASA I/II (No.) | 22/23 | 23/22 | 0.83/0.83 |
| Duration of the operation (min) | 121.9 ± 7.9 | 120.3 ± 6 | 0.28 |
Abbreviation: ASA, American Society of Anesthesiologists.
a Data are presented as mean ± standard deviation unless otherwise indicated.
b P > 0.05: Non-significant; P < 0.05: Significant; P < 0.01: Highly significant
c Using the Student’s t-test and chi-square test
4.2. Intraoperative Fluids Input, Urine Output, and Ephedrine Used
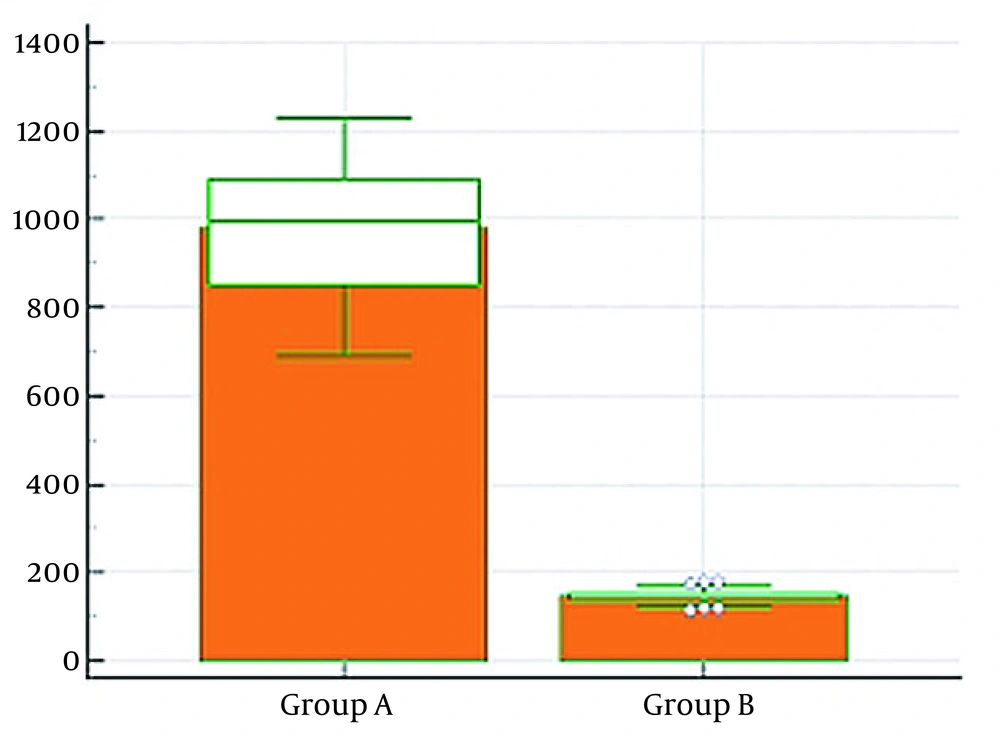
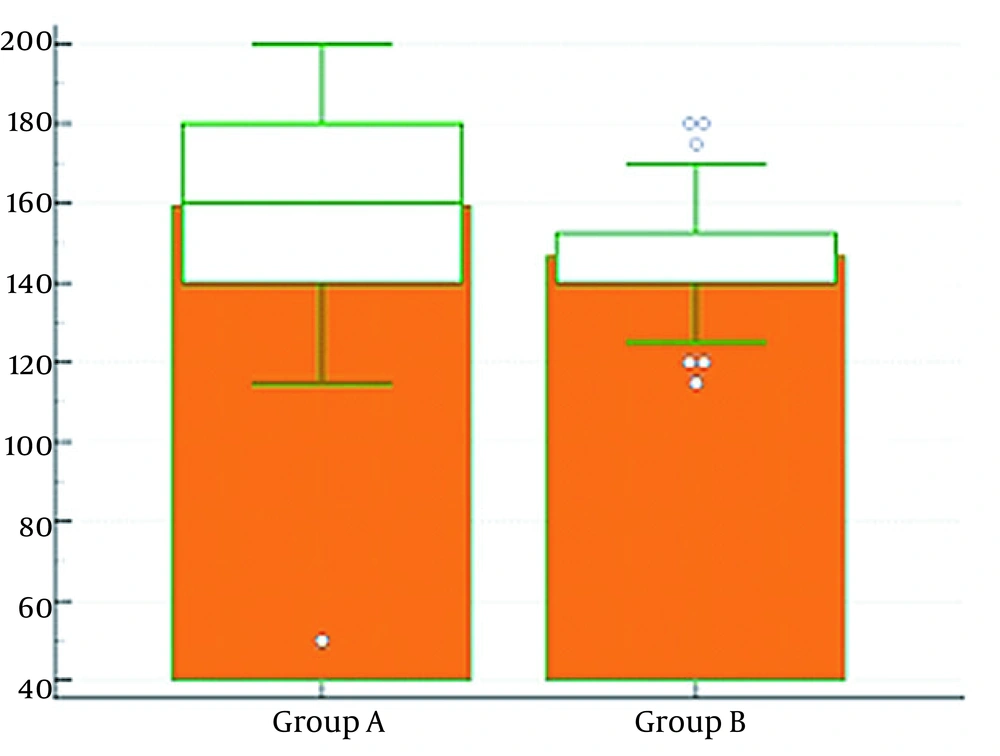
There was a highly significant statistical difference (P < 0.01) between the two groups in intraoperative crystalloid volume, higher in group A. Intraoperative urine output was significantly statistically lower in group B than in group A (P < 0.05). No significant statistical difference was noted between the two groups in the total amount of intraoperative colloids and ephedrine usage (P > 0.05), as shown in Table 2 (Figures 3 - 4).
| Variables | Group A | Group B | P-Value |
|---|---|---|---|
| Crystalloid (mL) | 979.4 ± 150.4 | 279.4 ± 38.7 | < 0.0001** |
| Colloids (mL) (No.) | 0 (0 - 0) (n = 9) | 0 (0 - 250) (n = 16) | 0.084 |
| Urine output (mL) | 159.2 ± 29.9 | 146.5 ± 15.8 | 0.013* |
| Ephedrine (mg) | 0 (0 - 0) | 0 (0 - 5) | 0.220 |
a Data are presented as mean ± standard deviation or median (25 - 75 percentile) unless otherwise indicated.
b P > 0.05: Non-significant; *P < 0.05: Significant; **P < 0.01: Highly significant
c Using the Student’s t-test and Mann-Whitney U test
4.3. Intraoperative Heart Rate and Mean Arterial Pressure
There was no significant statistical difference between the two groups in intraoperative heart rate and MAP (P > 0.05), as shown in Table 3.
| Variables | Group A | Group B | P-Value |
|---|---|---|---|
| HR 0 (beat per minute) | 79.3 ± 2.5 | 79.2 ± 2.9 | 0.86 |
| HR 1 (beat per minute) | 74.8 ± 3.6 | 74.7 ± 2.8 | 0.88 |
| HR 2 (beat per minute) | 74.8 ± 3.1 | 74.5 ± 2.4 | 0.60 |
| MAP 0 (mmHg) | 87.6 ± 5.2 | 86.9 ± 4.9 | 0.5 |
| MAP 1 (mmHg) | 79.6 ± 4.8 | 78.7 ± 3 | 0.28 |
| MAP 2 (mmHg) | 78.2 ± 5 | 78.7 ± 3 | 0.566 |
Abbreviations: HR, heart rate; MAP, mean arterial pressure.
a Data are presented as mean ± standard deviation.
b P > 0.05: Non-significant; P < 0.05: Significant; P < 0.01: Highly significant.
c Using the Student’s t-test.
4.4. Gravimetric Data
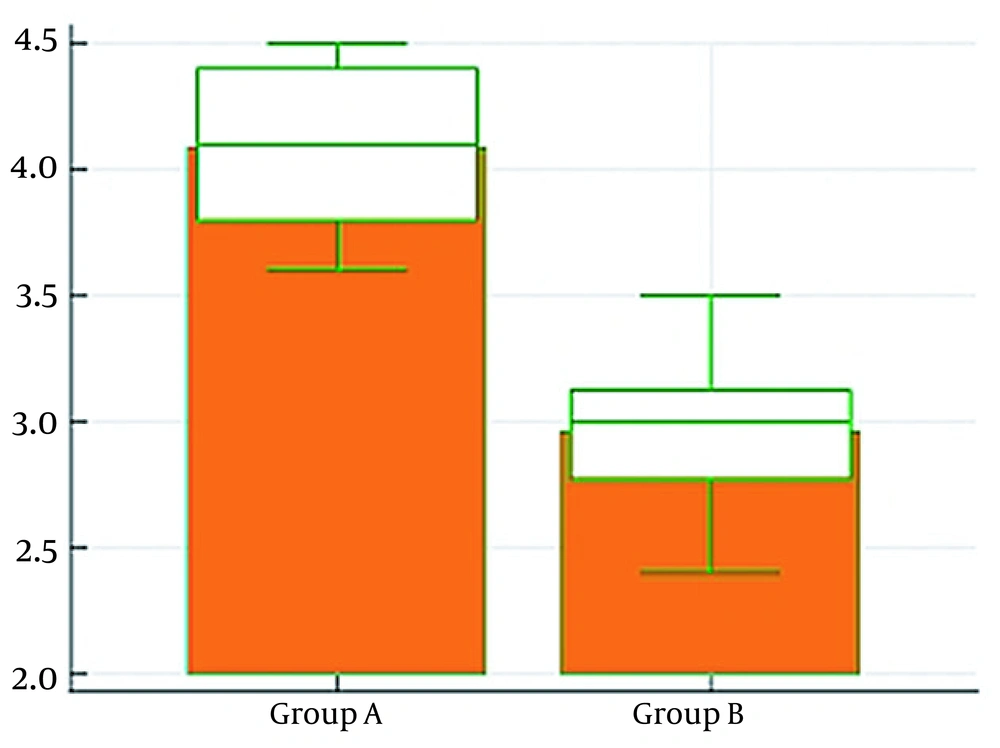
No significant statistical difference was observed between the two groups in the time from anesthesia induction to specimen removal (P > 0.05). Nevertheless, there was a highly significant statistical difference between the two groups in the water fraction of the bowel being higher in group A (P < 0.01), which showed higher bowel edema in group A than in group B (Table 4 and Figure 5).
| Variables | Group A | Group B | P-Value |
|---|---|---|---|
| Time from induction to specimen removal (min) | 79.9 ± 4.1 | 79.13 ± 8 | 0.56 |
| Water fraction (g) | 4 ± 0.29 | 2.9 ± 0.29 | < 0.0001** |
a Data are presented as mean ± standard deviation.
b P > 0.05: Non-significant; P < 0.05: Significant; **P < 0.01: Highly significant.
c Using the Student’s t-test.
4.5. Postoperative Outcomes
Postoperative bowel recovery and hospitalization period were significantly statistically higher in group A than in group B (P < 0.01). Furthermore, postoperative bowel anastomotic leakage was significantly higher in group A than in group B (P < 0.05) (Table 5).
| Variables | Group A | Group B | P-Value |
|---|---|---|---|
| Bowel recovery (day) | 4.5 ± 0.87 | 3.1 ± 1 | < 0.0001** |
| Anastomotic leak | 8 (17.7) | 2 (4.4) | 0.04* |
| Hospitalization period (day) | 5.7 ± 0.91 | 4.3 ± 1 | < 0.0001** |
a Data are presented as mean ± standard deviation or No. (%).
b P > 0.05: Non-significant; P < 0.05: Significant; **P < 0.01: Highly significant.
c Using the Student’s t-test and chi-square test.
5. Discussion
Pulse pressure variation-based GGFM was observed to be superior to CFM in patients treated with the resection of colon cancer mass and anastomotic surgery, according to the findings of the present study. Based on the results of this study, when PPV-based GGFM was compared to CFM in patients undergoing the elective resection of colon cancer mass and anastomotic surgery, the former reduced the intraoperative administration of total crystalloids, decreased the incidence of bowel edema, and shortened the time to bowel movements. Moreover, the patients with PPV-based GGFM were less likely to develop an intestinal anastomotic leakage and had shorter hospital stays.
Although extensive studies have been conducted on intraoperative fluid management, it is unclear what the proper fluid volume is. The only known scientific evidence demonstrates that excessive hydration might not be the best course of action (6).
Fluid administration is influenced by the patient’s age, anesthetic method, preoperative volume status, and concomitant conditions. Liberal fluid management (LFM) might be favored for low-risk patients undergoing high- and intermediate-risk surgery as opposed to restrictive fluid management (RFM) and generalized grading-scale fluid management (7).
Studies on hydration control during colorectal surgery have produced varying results. The primary issue is that the studies’ primary objectives differ. For instance, fluid management addresses postoperative problems and difficulties that RFM reduces. However, focused tissue perfusion and enhanced tissue perfusion were both successful (8). In contrast to LFM, RFM better preserved postoperative lung function, according to Holte et al. (9). However, Nisanevich et al. (10) observed that RFM decreased postoperative complications without affecting mortality. The findings of the aforementioned studies are in line with the findings of the current study.
The various definitions of intraoperative perfusion are another reason why the research outcomes varied. One source categorizes fluid management modes as CFM, RFM, and GGFM. Nevertheless, another source categorizes them as LFM, RFM, and GGFM (11). However, LFM and RFM are exempt from the requirement for uniform fluid volume by nature. For instance, Holte et al. (9). defined RFM as administering crystalloids and colloids simultaneously at 7 mL/kg/hour. Nevertheless, Abraham-Nordling et al. (12). defined LFM with crystalloids at 7 mL/kg/hour. The main objective of CFM is to replace losses during the intraoperative phase by fasting. However, after 10 hours of fasting, patients, even those without a cardiac risk, demonstrated euvolemia. One of the primary elements of CFM, a third space, is still up for debate. Consequently, hypervolemia might develop with CFM (13). Perioperative fluid excess in elective colorectal surgery has been linked to increased colon edema, renal diuresis, hindered defecation, postoperative ileus, decreased tissue oxygenation, and slowed wound healing due to increased skin edema (14).
The majority of GGFM trials have revealed favorable GGFM outcomes regarding respiratory risk, renal and gastrointestinal problems, time to bowel function recovery, and time to hospital discharge (11). When Pearse et al. (15) compared GGFM to CFM, they observed that although hospital stays were generally longer with GGFM, some problems, including surgical infections and 30-day mortality, were less common. Although bowel function returned sooner with GGFM in the current study, a statistical correlation could not be established between the time it takes to return after surgery and the length of hospital stay.
According to the evidence, 13.4% of patients who undergo major abdominal surgery might develop acute renal injury and might endure non-renal and prolonged postoperative problems. (16). In the present study, group B had a higher percentage of patients who experienced intraoperative oliguria.
It is possible to manage fluids using static, dynamic, invasive, or non-invasive parameters. Urinary output, blood pressure, and heart rate do not usually provide reliable data on volume status. If the patient’s heart rate, blood pressure, and urine output are all normal, they could be hypervolemic or hypovolemic (17). Although tachycardia is regarded as a classic sign of hypovolemia, the increased use of beta-adrenergic receptor antagonists in elderly individuals makes it difficult to accurately determine intravascular volume by heart rate (18).
Unless CVP is low (5 mmHg) and not a sign of clinical hypovolemia, intermittent CVP measures are of limited relevance (19). The efficacy of CVP measurement in fluid management is debatable because CVP thresholds are ambiguous, and measurements are affected by a variety of patient-related factors (20). According to Magder and Bafaqeeh’s study, high blood pressure is not a reliable indicator of the heart’s reaction to fluid management, and a CVP measurement of 10 mmHg might approximate euvolemia (21).
Although it has been discovered that dynamic measures, such as stroke volume variation (SVV), PPV, and systolic pressure variation utilized for GGFM, are preferable to static parameters in assessing fluid response, this cannot be proven. In the case of the near-maximal stroke volume -based GGFM group and the group aiming for zero balance and average postoperative weight, there were no appreciable changes in the postoperative results (22). Pulse pressure variation is considered more reliable than SVV and can be used to determine volume depletion before other signs (23).
Lopes et al. showed that PPV-guided intraoperative fluid therapy during high-risk surgeries improved postoperative outcomes and reduced the length of hospital stay (3). The results of the aforementioned study are completely consistent with the present study’s results.
Several studies have evaluated the effects of intraoperative fluid therapy driven by different hemodynamic targets, such as conventional fluid therapy, which is controlled by hemodynamic parameters, and fluid therapy, which is controlled by functional hemodynamic parameters, examined for the perioperative results. Studies have shown that patient outcomes have improved functional hemodynamic parameter-guided fluid therapy (PPV or SVV) during major surgical procedures. The aforementioned results are in line with the current study’s results.
In the present study, patients with ASA I-II alone might not be as dependable in cases with high ASA scores undergoing major surgery when large blood and fluid replacement is anticipated, in addition to the potential requirement for monitoring techniques. Inserting a CVP catheter is guidance for monitoring central venous oxygen saturation in these patients, which shows the balance between oxygen demand and supply.
5.1. Conclusions
In ASA I-II patients undergoing elective colorectal surgery, PPV-based GGFM, a minimally invasive intraoperative fluid management procedure, might be used instead of CFM as it enabled the researchers to use less intraoperative fluids without affecting patient intraoperative hemodynamics and urine output with less postoperative bowel edema, anastomotic leakage, rapid bowel recovery time, and shorter hospitalization period.
5.2. Strengths and Limitations of the Study
The strength of this study was in declaring the relationship between the intraoperative fluid amount and the incidence of bowel edema, leakage, bowel recovery, and hospital stay and highlighting the role of PPV-based GGFM in achieving that goal. On the other side, it was shown that the amount of intraoperative fluid volume was the study’s primary objective and that 45 patients were needed for each group. If postoperative problems and length of hospital stay were regarded as the study’s primary endpoints, the numbers might differ for each group. Another drawback was that the sample size was modest, and this study did not monitor the impact of PPV-guided hydration management on long-term patient prognosis and association with blood lactate levels. Patients should be monitored for a longer time following surgery, and postoperative problems could be examined in greater depth. Additionally, further studies are required to assess the efficacy and safety of PPV-based GGFM in high-risk patients and complex surgeries that might call for close monitoring.