1. Background
Unilateral Spinal Anesthesia (USpA) is a relatively popular method of anesthesia due to its asymmetric distribution with many positive characteristics (1-3). However, there is a high probability of spinal cord root damage (4), deviation of the needle from the midline (5), unsuccessful punctures, and absence of leakage of cerebrospinal fluid from the needle cannula (6). The frequency of successful unilateral blocks varies from 13 to 94% (7-12), which is certainly not a criterion for a "reliable" anesthesia method. Furthermore, the strict one-sided distribution of the anesthetic in the subarachnoid space is not always an achievable indicator (13), which varies from 68 to 94.45%. The aforementioned events lead to a high probability of multiple attempts to puncture the subarachnoid space, which either increases the risk of complications or leads to a complete failure with conversion to total intravenous anesthesia (TIVA). Repeated puncture attempts can cause the appearance of post-puncture headaches (PDPH) (14) due to considerable damage to the dura and, as a result, intracranial hypotension due to the loss of Cerebrospinal Fluid (CSF) through multiple dural defects (15).
Considering the absence of such research in the literature and the disadvantages of USpA, we proposed an original technique of unilateral spinal anesthesia using electrical nerve stimulation (USpA+ENS) (16-19). It allows for identifying the moment of dural puncture and the location of the spinal needle tip inside the subarachnoid space and towards the midline, directly affecting the development of the blockade.
2. Objectives
This work aimed to study and compare the efficacy and safety of conventional USpA and unilateral spinal anesthesia using electrical nerve stimulation (USpA+ENS).
Hereby, in the current study, we tested the hypothesis that the use of electrical nerve stimulation during USpA would: (1) reduce the rate of complications related to spinal punctures, such as PDPH, nausea, and vomiting, (2) increase the quality of anesthesia expressed in the VAS scale, Pin-prick and Bromage tests, lateralization of anesthesia and thereby’, patient satisfaction, and (3) simplify USpA and reduce the number of unsuccessful punctures.
3. Methods
3.1. Ethics and Trial Registration
This prospective, clinical, double-blind, randomized, parallel-group study was conducted after the approval of the Institutional Ethics Committee of Semey Medical University (28-09-2018/01) following the Declaration of Helsinki and the recommendations of the Consolidated Standards of Reporting Trials, registered in the UMIN database (UMIN000049554). All patients provided informed consent before the study. We conducted the research for six months, from October 2018 to March 2019.
3.2. Participants
One hundred forty-five patients aged 25 to 65 with upcoming operations for varicose veins of the lower extremities, mainly saphenectomy and crossectomy, with CEAP clinical classes from C2 to C4 and anesthetic risk according to ASA-I-IV, were enrolled in our study. Patients with ASA risk V, coagulopathy, acute cardiac and/or respiratory failure, hypovolemia, allergy or intolerance to local anesthetics, peripheral neuropathy, and mental disorders were excluded.
3.3. Sample Size
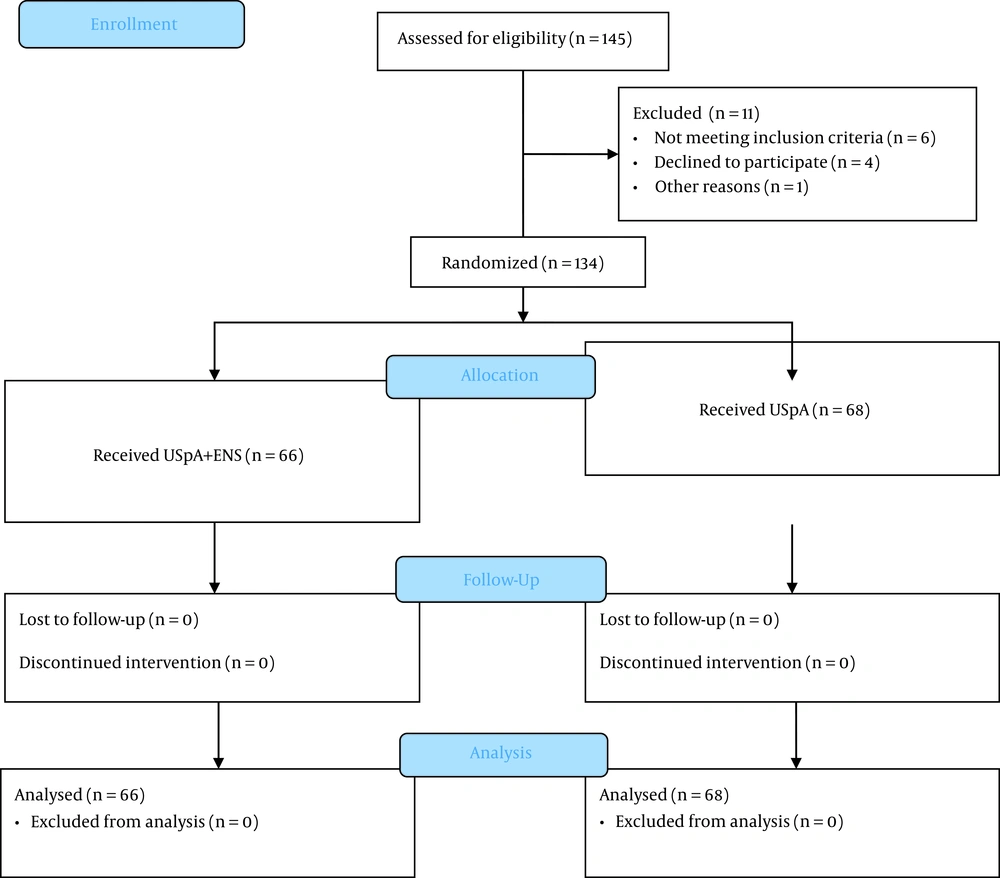
Based on the mean prevalence of varicose disease of the lower extremities of classes C1-C4, from 25 to 30% (20), we adopted a 28% population prevalence. Power analysis showed a prevalence of 28% with a significance level of 0.05 and 10% of possible dropout within a power value of 80%; hence, a sample size of at least 130 (65 per group) was calculated. The calculations were made with GPower® 3.1 based on a t-test for independent groups with common variance. Figure 1 shows the recruitment of study participants.
3.4. Randomization and Allocation Concealment
Patients were randomly assigned to one of two study groups. Random tables were generated using SPSS® 20.0. According to the sample size, a statistician who did not participate in the study prepared one hundred and forty-five envelopes in advance. Patients or observers were unaware of group assignments. The allocation sequence was concealed from the researchers enrolling and assessing participants using opaque, sealed, and sequentially numbered envelopes opened by an anesthesiologist who was not involved in this study. Observation in the early postoperative period, including the registration of complications, was carried out by a third-year trainee resident who did not participate in the study. Therefore, investigators who directly performed the anesthesia were independent of those responsible for statistical processing.
3.5. Outcome Measures
The level of sensory blockade was assessed using the 'pin-prick' test, while the motor blockade level was evaluated according to the modified Bromage scale. Evaluation of pain level was carried out according to the Visual Analogue Scale (VAS). Spinal anesthesia was considered unilateral if the sensory block at 20 minutes was at the Th12-L1 level and the modified Bromage score was > 2 in the operated limb.
The adequacy of anesthesia was determined by no need for additional administration of analgesics and anesthetics, i.e., inadequate required only the administration of analgesics, and anesthesia failure if there was a transition to general anesthesia (TIVA). Lumbar puncture was defined as 'successful' on the first attempt if the cerebrospinal fluid was obtained during the first puncture.
The overall follow-up of the patients was 3 to 11 bed days, depending on the postoperative period and the presence of complications, with an average of seven bed days. Thus, we limited the observation of patients to the early postoperative period, since all the main complications of anesthesia developed on the second and third day after the surgery, and there was no need for the long-term follow-up of the patients
3.6. Interventions
The USpA technics: Patients were preloaded with 6 - 8 mL/kg of crystalloids before surgery. The patient was placed in the lateral decubitus position on the surgery side. After Local Infiltration Anesthesia (LIA) of the skin with 4 - 5 mL of 0.5% Novocain solution, the subarachnoid space was punctured at the level of LIII-LIV with a standard 27G Quincke cut spinal needle with the introducer. After puncturing the dura mater and obtaining cerebrospinal fluid out of the needle cannula, the needle cut was turned down, and then 7.5 µg of 0.5% Bupivacaine-Spinal® was injected in 100 - 120 seconds. After injection, the patients remained in the lateral decubitus position for 15 - 20 minutes.
USpA+ENS technics: Similar to the USpA method, patients were preloaded with 6 - 8 mL/kg of crystalloids. The patient was placed in a lateral decubitus position on the surgery side. After LIA of the skin with 4 - 5 mL of a 0.5% Novocain solution, the epidural space was punctured with a resistance loss test at the LIII-LIV level with a Stimuplex® 22G 50 mm needle, connected to Stimuplex-HNS 12 for electrical nerve stimulation. A current of 4 mA with a frequency of 2 Hz and pulse duration of 0.1 ms was set. In the case of a clear resistance loss test, indicating that the needle tip was in the epidural space, a classic Quincke cut 29G 90 mm spinal needle was administered through the Stimuplex 22G needle. Immediately after the dural puncture, the current, passing through the inner wall of the needle for electrical nerve stimulation to the spinal needle, reached the subarachnoid space. The patient felt the sensation of irritation with an electric current and immediately reported it to the anesthesiologist. Furthermore, the anesthesiologist visually observed the effect of electric current in the form of a motor response; in particular, muscle contractions were observed in the necessary limb. In the case of electrical stimulation, the free flow of CSF was also observed through the spinal needle.
The presence of these two criteria confirmed the exact location of the needle tip in the subarachnoid space. The needle cut was then turned down, and 7.5 µg of 0.5% Bupivacaine-Spinal® was administered in 100 - 120 seconds. After injection, patients remained in the lateral decubitus position for 15 - 20 minutes.
3.7. Statistics
Data analysis was done using SPSS 20.0 software (IBM Corp, Armonk, N.Y, USA). The confidence interval was 80%, with a two-sided significance of 0.05 at a power of 80%. The normality of the distribution was checked using the Shapiro-Wilk test, while the Levine test checked the equality of the variances. Patient characteristics and hemodynamic parameters were analyzed by Student's t-test and Mann-Whitney U test. The safety parameters of anesthesia, intraoperative correction of hemodynamics, and additional analgesia were processed by the chi-square test. Most of the quality parameters of anesthesia were analyzed using Kendall's Tau. Quantitative data were presented as the arithmetic mean ± standard deviation (SD). Ordinal data were presented as median (interquartile range).
4. Results
In total, 145 patients were enrolled in this study. Six patients did not meet the inclusion criteria, four refused to participate in the study, and one was excluded due to inappropriate follow-up. Thus, a total of 134 patients were included. Almost half of them, namely 68 patients, were assigned to the USpA (control) group, and the remaining 66 patients were assigned to the USpA+ENS (main) group (Figure 1).
The baseline demographic data of the patients involved in the study, such as mean age, height, weight, BMI, gender, ASA risk, and general clinical condition expressed in terms of respiration and hemodynamics (SBP, DBP, heart rate, and SpO2) did not differ significantly between the groups (Table 1).
| USpA | USpA+ENS | P-Value | Criterion | |
|---|---|---|---|---|
| Age, y | 52.51 ± 13.01 | 54.47 ± 11.78 | 0.364 | t-Student |
| Height, cm | 165.91 ± 8.43 | 166.23 ± 7.60 | 0.821 | t-Student |
| Weight, kg | 76.66 ± 18.58 | 77.98 ± 14.75 | 0.649 | t-Student |
| BMI | 27.73 ± 5.82 | 28.23 ± 5.13 | 0.599 | t-Student |
| Gender | 0.554 | χ2-test | ||
| Male | 26 (38.2) | 22 (33.3) | ||
| Female | 42 (61.8) | 44 (66.7) | ||
| Risks based on ASA | 0.334 | Tau-C | ||
| 1 | 9 (13.2) | 6 (9.1) | ||
| 2 | 47 (69.1) | 45 (68.2) | ||
| 3 | 12 (17.6) | 15 (22.7) | ||
| Mean SBP | 124.21 ± 18.43 | 128.87 ± 15.09 | 0.112 | t-Student |
| Mean DBP | 81.59 ± 12.44 | 81.41 ± 9.85 | 0.733 | Mann-Whitney |
| Heart rate | 73.53 ± 12.22 | 73.82 ± 12.33 | 0.631 | Mann-Whitney |
| SpO2 | 97.93 ± 1.05 | 97.81 ± 1.41 | 0.682 | Mann-Whitney |
| Duration of operation | 60.00 ± 22.95 | 57.80 ± 1.05 | 0.506 | Mann-Whitney |
Initial Demographic Data and General Clinical Condition
Full anesthesia of the operated limb was achieved in 57 patients versus 48 patients in the control group. Similar superiority was shown for USpA+ENS over the control group in the modified Bromage test (54 vs. 42 cases; P = 0.014) and the VAS scale (51 vs. 36 cases; P = 0.002). Also, the lateral spread of anesthesia (USpA/BSA) was superior in the main group (60 vs. 51 cases; P = 0.015). The USpA+ENS group showed better patient satisfaction with VAS (51 vs. 36 cases; P = 0.002), and the adequacy of anesthesia was also superior in the main group (60 vs. 51 cases; P = 0.038) (Table 2).
| USpA, No. (%) | USpA+ENS, No. (%) | P-Value | Criterion | |
|---|---|---|---|---|
| Pin-Prick test | 0.018 | Tau-C | ||
| Pain sensitivity preserved | 3 (4.4) | 0 (0) | ||
| Analgesia (blunt touch without pain) | 17 (25) | 9 (13.6) | ||
| Anesthesia (lack of sensation) | 48 (70.6) | 57 (86.4) | ||
| Bromage test | 0.014 | Tau-C | ||
| Raise of the straight leg | 2 (2.9) | 0 (0) | ||
| Raise of the leg bent at the knee | 6 (8.8) | 6 (9.1) | ||
| Movements only in the ankle joint | 18 (26.5) | 6 (9.1) | ||
| Full motor block | 42 (61.8) | 54 (81.8) | ||
| Patient satisfaction (VAS) | 0.002 | Tau-C | ||
| No pain (0) | 36 (52.9) | 51 (77.3) | ||
| Good (1 - 2) | 15 (22.1) | 8 (12.1) | ||
| Bearable (3 - 4) | 9 (13.2) | 4 (6.1) | ||
| Painful (5 - 6) | 7 (10.3) | 3 (4.5) | ||
| Very painful (7 - 8) | 1 (1.5) | 0 (0) | ||
| Unbearable pain (9 - 10) | 0 (0) | 0 (0) | ||
| Lateralization of anesthesia (USpA/BSA) | 0.015 | χ2-test | ||
| Unilateral anesthesia | 51 (75) | 60 (90.9) | ||
| Bilateral anesthesia | 17 (25) | 6 (9.1) | ||
| Adequacy of anesthesia | 0.038 | χ2-test | ||
| Adequate anesthesia | 51 (75) | 60 (90.9) | ||
| Inadequate anesthesia | 15 (22.1) | 6 (9.1) | ||
| Failed Anesthesia/TIVA Conversion | 2 (2.9) | 0 (0) |
Details of Anesthesia Quality
The safety of anesthesia we assessed by the incidence of nausea, vomiting, post-puncture headache, and the number of attempts to puncture the dura mater. These criteria, except vomiting, were significantly less frequent in the main group (Table 3).
| USpA, No. (%) | USpA+ENS, No. (%) | P-Value | Criterion | |
|---|---|---|---|---|
| Nausea | 0.031 | χ2-test | ||
| Presence | 14 (20.6) | 5 (7.6) | ||
| Absence | 54 (79.4) | 61 (92.4) | ||
| Vomiting | 0.120 | χ2-test | ||
| Presence | 4 (5.9) | 0 (0) | ||
| Absence | 64 (94.1) | 66 (100) | ||
| PDPH | 0.028 | χ2-test | ||
| Presence | 6 (8.8) | 0 (0) | ||
| Absence | 62 (91.2) | 66 (100) | ||
| Number of attempts | 0.045 | Mann-Whitney | ||
| 1 | 58 (85.3) | 63 (95.5) | ||
| 2 | 8 (11.8) | 3 (4.5) | ||
| 3 | 2 (2.9) | 0 (0) |
Details of Anesthesia Safety
The overall volume of intravenous infusion was slightly lower in the USpA+ENS group than in the control group (Me = 800 vs. Me = 850; P = 0.122). Additional analgesia was also less frequent in the main group (P = 0.031), while the correction of hemodynamics did not differ significantly (Table 4).
| USpA, No. (%) | USpA+ENS, No. (%) | P-Value | Criterion | |
|---|---|---|---|---|
| Volume of intravenous infusion | Me = 850 | Me = 800 | 0.122 | Mann-Whitney |
| Correction of hemodynamics | 0.078 | χ2-test | ||
| Not carried out | 54 (79.4) | 61 (92.4) | ||
| crystalloids up to 1000 mL | 1 (1.5) | 1 (1.5) | ||
| Crystalloids over 1000 mL | 4 (5.9) | 0 (0) | ||
| Colloids up to 1000 mL | 9 (13.2) | 3 (4.5) | ||
| Colloids over 1000 mL | 0 (0) | 1 (1.5) | ||
| Additional analgesics | 0.031 | χ2-test | ||
| Not carried out | 47 (69.1) | 59 (89.4) | ||
| NSAIDs | 6 (8.8) | 3 (4.5) | ||
| Synthetic analgesics. hypnotics | 6 (8.8) | 1 (1.5) | ||
| Narcotic analgesics (promedol, morphine, fentanyl) | 9 (13.2) | 3 (4.5) |
Details of Hemodynamic Correction
5. Discussion
There were no significant differences in the baseline demographic data for the two study groups. Along with this, there were no statistically significant differences in hemodynamic parameters due to the comparison of two technically similar methods of anesthesia (UsPA and USpA+ENS) with the same doses of local anesthetics but differing in the use of electrical nerve stimulation (Table 1).
The USpA+ENS technique is based on the high electrical conductivity of cerebrospinal fluid that immediately transmits the electric current from the needle tip to nearby nerve roots, right after the dura mater puncture (21, 22). That is, no further passage of the needle is required due to muscle contractions of the required limb and the patient's sensations of irritation by the electric current. Thereby, an anesthesiologist knows that the needle tip has punctured the dura and is located directly in the subarachnoid space. Moreover, the outflow of CSF from the lumen of the spinal needle became an additional criterion, while it is still the main one for any spinal anesthesia performed without electrical nerve stimulation (23, 24). Thus, indicators of the quality of anesthesia, namely Pin-prick and Bromage tests, the VAS scale, and others were superior in the USpA+ENS group (Table 2).
Other challenges faced during the spinal puncture include a significant reduction in CSF pressure while performing the USpA in the side-lying position and 29G needles usage with a small lumen lengthening the time for CSF to appear in the needle pavilion up to 20 seconds (25). Likewise, anesthesiologists usually focus on the sensation of "falling" and "clicking" when puncturing the dura, which may be different in strength depending on the type of needle tip and its diameter (26).
This study showed the relationship between the number of attempts at dura mater puncture and the incidence of adverse events, such as PDPH, and its consequences (27). Considerable damage to the dura can be worsened with a high probability of needle tip deformation when using 27G and 29G needles, leading to further CSF loss. The most formidable is the deformation of the needle tip in the form of a hook, and the frequency of this event is slightly reduced with the use of an introducer but is not entirely excluded (28, 29). In our case, based on the USpA+ENS technique, first, an isolated Stimuplex® needle for neurostimulation with a 60-degree cut was used to puncture the epidural space with a resistance loss test. Only after that, a 29G needle was passed through the first needle, which cannot face any obstacle leading to its deformation, and it remains only to puncture the dura.
These challenges restrain the conduct of the USpA by anesthesiologists. However, the implementation of ENS helps to avoid these factors confirmed by the safety indicators of spinal puncture, namely the incidence of PDPH, nausea, vomiting, and the number of puncture attempts surpassed in the main study group (Table 3). Using a combination of needles for electrical nerve stimulation and Quincke 29G 90 mm spinal needles allows one to figure out the location of the needle tip in the subarachnoid space relative to the midline, thereby, more accurately injecting a local anesthetic into the subarachnoid space; on the other hand, it minimizes the likelihood of complications of dural puncture in the form of PDPH.
Thus, taking into account the factors mentioned above, we accept the hypothesis of this study regarding the quality, safety, and simplicity of anesthesia. These valuable results make it possible to identify a further research direction, comparing peripheral nerve blocks of the lower extremities, USpA, and USpA+ENS with ultrasound navigation.
5.1. Limitations and Strengths
The main limitations of this study were the use of 27-gauge needles for the USpA group, while for the main group, 29-gauge needles were used. Also, the patient's follow-up was limited to an average of 11 days of hospitalization, so there was no long-term monitoring of patients for possible complications.
The main strength was that there were no similar studies on implementing electrical nerve stimulation during unilateral spinal anesthesia. Our earlier patents on identifying the subarachnoid space were the basis for the work done.
5.2. Conclusions
The technique of unilateral spinal anesthesia with electrical nerve stimulation allows objectifying the entire anesthesia process, from the epidural and subarachnoid space puncture to obtaining a blockade on the side required for surgical intervention. This technique has a high selectivity in the spread of the spinal block, making it possible to correct the endpoint of the needle tip injection and increase the safety of anesthesia, its quality, and commitment for patients with different comorbidities.