1. Background
Chronic low back pain is one of the most common medical problems in the western world. A lot of different treatment options are suggested for (chronic) low back pain (1). One of the treatment options that receives a lot of attention nowadays is spinal cord stimulation (SCS). SCS has shown to be an effective therapy for chronic neuropathic pain conditions such as failed back surgery (FBSS). Since its introduction in 1967 (2), the working mechanism of SCS has always been based on the induction of paresthesia. Paresthesia was considered necessary and had to cover the painful area, as well as possible, to achieve maximal pain relief (2-5). But recent research postulated that paresthesia may not be necessary for pain relief and may even offset the clinical benefits of SCS by increasing pain awareness (6) and/or causing paresthesia discomfort (7). Based on these findings, new, subthreshold stimulation algorithms have been introduced to optimize pain control. Those algorithms attempt to achieve paresthesia-free stimulation, aim at new stimulation targets, and/or use alternate waveforms (8-15).
High-density (HD) stimulation is one of those promising, new stimulation paradigms, involving an increase in frequency and pulse width, along with a reduced amplitude, when compared to conventional SCS (16, 17). Although promising results with HD-SCS have been reported (18), these findings need to be confirmed by a larger study with a longer follow-up period.
High density in spinal cord stimulation: virtual expert registry (DISCOVER) is designed to investigate the effectiveness, safety, and feasibility of HD-SCS in a population of FBSS patients with chronic back and leg pain.
2. Methods
2.1. Study Design
DISCOVER (ClinicalTrials.gov: NCT02787265) is a prospective, multi-center, clinical study evaluating the effectiveness of HD-SCS in patients with FBSS. For the purpose of this study, HD stimulation parameters are defined for each device (See Table 1). All patients included in this study have an IPG of Medtronic (Minneapolis, MN, USA), either rechargeable (RestoreADVANCED®, RestoreSensor®) or battery powered (PrimeADVANCED®).
| Devices | ENS | RS | PA/RA | |
|---|---|---|---|---|
| Programs | A1 | A1 | A1 | A2 |
| Pulse width, µsec | 800 | 500 | 450 | 450 |
| Frequency, Hz | 300 | 500 | 130 | 130 |
| Pulse density, % | 24 | 25 | 11.7 | |
HD Parameters; ENS: External Neurostimulator; RS: RestoreSensor; PA/RA: PrimeAdvanced/RestoreAdvanced
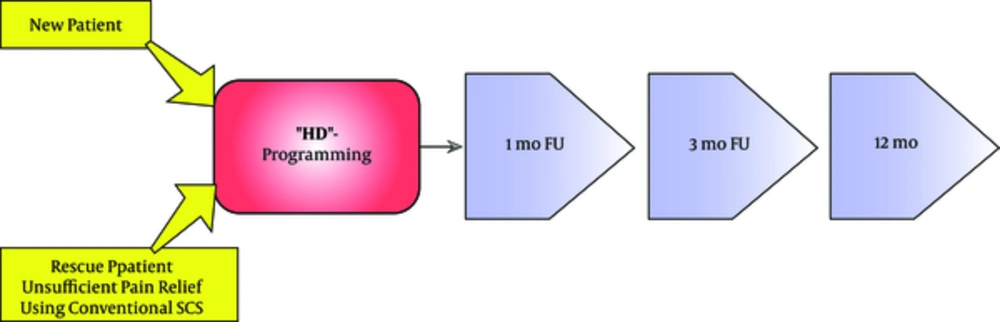
No patient will be randomized because all patients receive HD-SCS as part of good clinical practice (GCP), nor are subjects or physicians blinded to the received stimulation. Prior to enrollment, all subjects sign an informed consent (IC). As soon as IC is provided, patients undergo a baseline visit. For a rescue patient, the baseline evaluation occurs on the day their conventional SCS settings are converted to HD settings. The baseline evaluation of a new patient marks the beginning of their trial phase (4 weeks, according to Belgian legislation) with an external neurostimulator (ENS); after a successful trial these patients will get an implantable pulse generator (IPG) and proceed to the next phase of the study. The type of electrodes used will be chosen by the implanting physician according to their expertise and the patient’s needs.
From here on, all patients have a standardized one year follow-up period based on three out-patient visits (one month, 3 months, and 12 months) (Figure 1).
The recruitment period of DISCOVER is at least 12 months, unless the goal of 400 patients is reached earlier. Patient recruitment began in October 2016 and is projected to end by October 2017 (last patient in). Recruitment may continue afterwards until the aforementioned target of 400 patients is reached. Follow-up is expected to end by October 2018 (last patient out).
2.2. Study Population
Patients (≥ 18 years) with chronic back and leg pain (i.e. > 6 months with a pain intensity of > 5 NRS) due to FBSS are eligible for this study (see Table 2). Exclusion criteria (see Table 3) are: lupus erythematosus, morbus bechterew, coagulation disorders or a life expectancy < 1 year. Patients that suffer from any addiction to drugs, alcohol and/or medication are also excluded. Reflecting daily medical practice, DISCOVER allows participation of neurostimulation-naïve “new patients” and “rescue patients”.
| Criteria | |
|---|---|
| 1 | Failed back surgery syndrome (FBSS) patients with insufficient pain relief through conventional spinal cord stimulation (SCS): (“rescue patient”) |
| 2 | FBSS patients who are suited for SCS (“new patient”) |
| 3 | Age > 18 years |
| 4 | Chronic pain as a result of FBSS that exists for at least 6 months with a pain intensity of 5 (or higher) on the NRS |
Inclusion Criteria
| Criteria | |
|---|---|
| 1 | Expected inability of patients to receive or properly operate the SCS system |
| 2 | History of coagulation disorders; Lupus erythematosus; diabetic neuropathy; rheumatoid arthritis; Morbus Bechterew; Active malignancy; immune deficiency |
| 3 | Life expectancy < 1 year |
| 4 | Addiction to drugs, alcohol (5 units/day) and/or medication |
Exclusion Criteria
2.3. New Patients
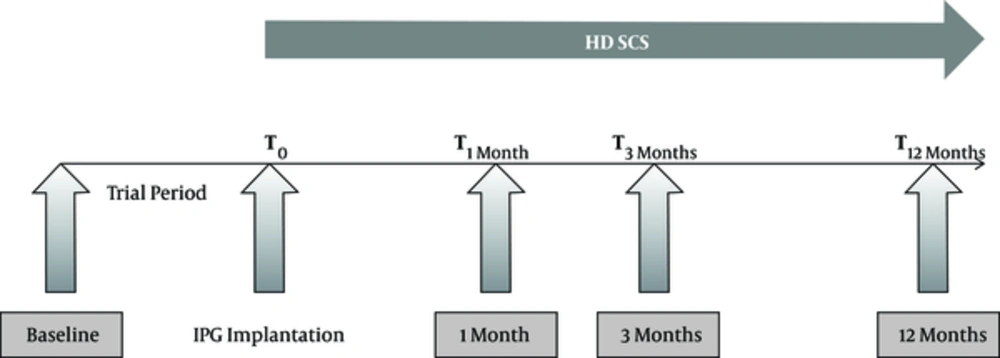
These neurostimulation-naïve patients are suited for SCS according to good clinical practice (GCP). Baseline evaluation will be performed prior to lead implantation, as shown in Figure 2. The study takes a SCS-trial period of 4 weeks into account with an external neurostimulator (ENS), in compliance with the Belgian reimbursement rules. Conventional stimulation and HD stimulation settings may be tested during this trial period. After a successful trial, according to Belgian legislation, the ENS will be replaced by an IPG. Subsequently patients will receive HD-SCS during 1 year, and will be assessed at 1, 3, and 12 months with an assembled series of questionnaires (Table 4).
| VISITS | ||||
|---|---|---|---|---|
| Screening | High Density | |||
| V0 | V1 | V2 | V3 | |
| Inclusion/Exclusion criteria | X | |||
| Analgesic medication | X | X | X | X |
| Pain map | X | X | X | X |
| Pain NRS | X | X | X | X |
| ODI | X | X | X | X |
| EQ-5D | X | X | X | X |
| PSQI | X | X | X | X |
| Paresthesia map | X(*) | |||
| MRI scanning | X | X | X | |
| SCS settings & parameters | X | X | X | X |
| PAM-13 | X | X | X | X |
Questionnaires Collected on Baseline Visit (V0), 1 Month (V1), 3 Months (V2), and 6 Months (V3); (*) = Only Rescue Patients
2.4. Rescue Patients
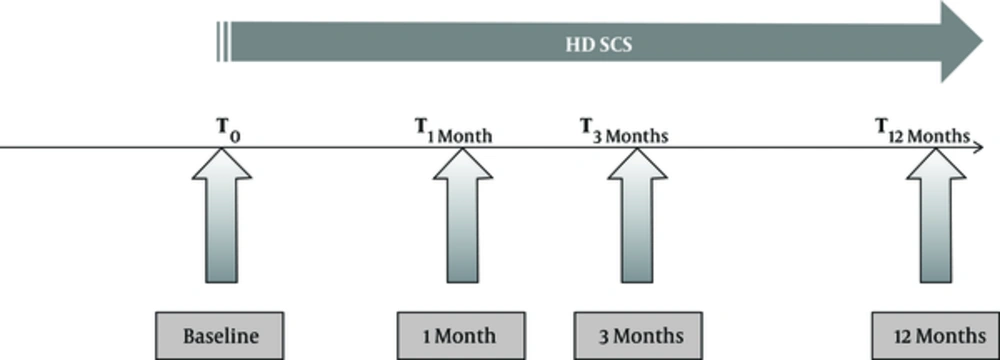
Rescue patients are patients in whom the initial effectiveness of conventional SCS is lost over time and/or those who experience the induced paresthesia as too painful. In these cases, HD-SCS can be an alternative treatment option. A rescue patient is defined according to the following criteria: numerical rating scale (NRS) > 3/10 for leg and/or back pain AND a Likert scale score of “not at all satisfied” or “slightly satisfied” with conventional SCS as their treatment (Appendix 1.1). Following conversion from their current conventional SCS parameters to HD-SCS (baseline visit, T0), the patient can be enrolled in the DISCOVER study (Figure 3).
2.5. Study Outcome Measures
Timelines and a general flowchart of DISCOVER are shown above (see Figures 1 - 3). Each visit will contain several questionnaires, as listed in Table 4 (19). After recruitment, a one-year follow-up will start where patients’ pain ratings, sleep quality, disability, satisfaction, and complication rates will be assessed. Along with primary outcomes (clinical effectiveness, safety and feasibility), secondary outcome measures that will be noted are: PSQI, EQ-5D, ODI, pain medication, pain and paresthesia mapping.
2.6. Primary Outcome Measures
2.6.1. Clinical Effectiveness
The primary measure for the evaluation of clinical effectiveness of HD-SCS is the mean reduction in pain intensity. This relative clinical improvement will be defined by the following formula: (NRSHD-NRSbaseline)/NRSbaseline. Secondary endpoints that will be used to measure the clinical effectiveness of HD-SCS include assessments of the quality of life (EQ-5D), disability (ODI), quality of sleep (PSQI), and an overall reduction in used pain medication.
2.6.2. Safety
Morbidity 12 months after initiating HD-SCS was chosen as the primary safety endpoint of DISCOVER. Furthermore, we will register any adverse event (AE), serious adverse event (SAE), and suspected unexpected adverse reaction (SUSAR) that may be related to the stimulation paradigm.
2.6.2. Feasibility
As a measure for the feasibility of HD-SCS, the following endpoints were chosen: proportion of successful HD trials, the prevalence of technical issues, and the battery usage.
2.7. Secondary Outcome Measures
2.7.1. Pain intensity and Medication
To register pain intensity, an 11-point numerical rating scale (NRS) will be used, which will be taken at each visit (baseline, 1, 3, and 12 months). Participants will be asked to rate their back and/or leg pain from 0 to 10, with 0 equaling no pain and 10 representing the worst imaginable pain (20). During each follow-up visit, we will keep track of the pain medication used by each individual patient, the average morphine equivalent dosage will be registered.
2.7.2. Pain and Paresthesia Mapping
Pain location is documented on a body map where different segments of the body are represented in a standardized manner. Patients can indicate their painful body segments by writing down the corresponding numbers. The zone in which paresthesia is felt will also be documented, using a similar standardized body map; this will only be asked on the baseline visit of rescue patients.
2.8. Pittsburgh Sleep Quality Index (PSQI)
Sleep quality will be assessed by means of Pittsburgh sleep quality index (PSQI) (21). The PSQI is a validated scale that provides a standardized, quantitative measure of sleep quality that quickly identifies good and poor sleepers. 19 individual items generate 7 component scores, the sum of these 7 component scores gives one global score to distinguish between good and poor sleepers.
2.9. Oswestry Low Back Pain Questionnaire (ODI)
The Oswestry low back pain questionnaire is widely used for determining functional disability due to low back pain (22). This questionnaire contains 10 topics concerning intensity of the pain, lifting, ability to care for oneself, ability to walk, sit and stand, sexual function, social life, ability to travel, and sleep quality. Each category is followed by 6 statements and each statement is scored on a scale of 0 - 5. The scores of all questions are summed and multiplied by 2 to obtain the index (0 - 100). 0 is no disability and 100 is the maximal disability possible.
2.10. EQ-5D-3L
The EQ-5D records physical, psychological, and social aspects of health to describe the overall health status of a patient (23). The questionnaire is formed by 5 dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Patients subjectively tick the box of the most appropriate statement in each dimension. These questions are followed by a 20 cm, vertical VAS scale from 0 to 100, where 100 is labeled as “the best health you can imagine”, and 0 as “the worst health you can imagine”.
2.11. SCS Parameters and Settings
In each visit, the investigator will be asked to report the used SCS settings and parameters. The parameters that will be registered are: frequency, pulse width, voltage, and active electrodes.
2.12. PAM-13
Participation and engagement play an important role in the healing process of the chronically ill patients. A lack of engagement can be one of the causes of treatment failure. The PAM-13 or patient activation measure (PAM) is a 13-item instrument which assesses self-reported behavior, knowledge, and confidence for self-management of one’s health. PAM- 13 has proven to be a reliable instrument to measure patient activation and self-management (24). Patients will be divided in 4 levels, going from disengaged with lack of confidence (level 1) to individuals who maintain their healthy lifestyle and feel confident about their health (level 4).
2.13. Center Eligibility
DISCOVER is currently restricted to Belgium where 19 neuromodulation centers with ample SCS and HD-SCS experience were selected, with the aim to recruit approximately 20 patients each. All centers had to meet the following minimum criteria: first and foremost they should have the required knowledge of HD-SCS (clinical and programming) with at least 5 - 10 patients treated. Second, each center should have the ability to recruit 15 - 20 patients with HD SCS in 12 months, consisting of approximately 7 - 10 rescue patients and new patients. To conclude, each center should have the necessary tools and resources available to collect and report all study-related data on a web-based data-collection platform.
2.14. Lost to Follow-Up
In case a patient is lost to follow-up, attempts will be made to contact the patient by phone to perform any further assessment. These will only be performed after explicit agreement of the patient.
When HD is not effective or not preferred by the patient, stimulation can be reverted to conventional SCS settings. The reasons for change in stimulation settings will be documented and follow-up will take place according to the protocol, if the patient agrees.
2.15. Statistical Analysis and Data Management
Analyses will be conducted on data collected from all registered patients. The sample size is based on our clinical experience in Belgium. The study goal is to estimate the percentage of patients who would respond to HD SCS. With a sample size of 404 patients, we will be able to estimate this value with an error margin of 5 percentage points with an expected rate of responders at 70% and assuming 20% of missing data. The main analysis of DISCOVER consists of a single effect comparison of the primary outcome measures between baseline and clinical status at 1 month, 3 months, and 12 months. The primary effectiveness endpoint is defined as the relative clinical improvement (pain intensity, functional outcome, sleep, and pain medication use) of patients with FBSS using the NRS, EQ-5D, and PSQI. The primary effectiveness endpoint is defined as the relative proportional clinical improvement (pain intensity) on the NRS, defined as: (NRSHD-NRSbaseline)/NRSbaseline. For all our analyses a P value of 0.05 will be used.
Personal data will be processed in accordance with the European Union’s data protection directive (Directive 95/46/EC) and regulation EC45/2001, the relevant Belgian legislation, and good practice. Data will only be processed for trial purposes. Person-identifiable data will not leave the unit from which they originated, and keys to identification numbers will be held confidentially within the clinical unit. For primary data-collection, the Asklepios platform is used, a proprietary data-collection software tool developed at the Vrije Universiteit Brussel (VUB). Data will be recorded in an electronic case record form (e-CRF) provided by OpenClinica LCC, Waltham, United States of America, and stored in a secured digital database. All individual patients’ data will be linked to the e-CRF via a unique identification number (i.e. subject number). Individual patient’s medical information will be recorded and transferred for analysis only in anonymized form.
2.16. Ethical Considerations
Approval for the conduct of the trial has been obtained from the ethics committee of the Universitair Ziekenhuis Brussel, the ethics committee of each participating center, and from the regulatory authorities. The trial will be conducted according to the principles laid down in the declaration of Helsinki (Seoul version, 2008), the European Union clinical trial directive 2001/20/EC, the “Note for guidance on good clinical practice” (CPMP/ICH/135/95 of January 17th 1997), and the GCP-regulation from August 9th 2004.
The design of the trail has been carefully reviewed and approved by the steering committee of DISCOVER before being submitted for approval by the ethics committee and Regulatory Authorities. In addition, the independent external scientific advisory board (SAB) and data safety monitoring board (DSMB) have reviewed and approved the trial protocol and will continuously monitor the conduction of the trial.
The registry can be halted or terminated in any case of concern for the safety of the patients resulting from new information. The DSMB will continuously monitor the rate of SAE and SUSAR and will have the ability to stop the trial in any case of unexpected high or alarming rates of SAE or SUSAR. Trial registration: NCT02787265.
3. Discussion
Traditionally, conventional SCS focuses on paresthesia coverage of the painful area, thereby activating the large diameter sensory fibers which play an important role in pain relief (25). Nowadays, we know that axonal activation is not the only effect produced by SCS. Several other physiological effects have already been described: alterations in wide dynamic range neuronal membrane excitability, changes in release of neurotransmitters, activation and/or inhibition of various descending and ascending pain pathways (26, 27). Because of these insights, the hypothesis that different SCS patterns may activate unique pain-relieving mechanisms has emerged. Different SCS settings may offer specific therapeutic approaches which: 1) offer superior pain relief, 2) provide additional choices of therapy for patients, and/or 3) target more specific mechanisms underlying a pain syndrome (28). Furthermore, a recent study stated that an increase in pulse duration, with a higher electric charge, has a greater effect on the effectiveness of SCS than variations of pulse shape. It shows that the choice of electrical parameters is an often overlooked factor, contributing to SCS effectiveness (17).
It is important to note that HD-SCS may suffer from some drawbacks. First of all, from a safety perspective, conventional SCS is regarded as a safe method of treatment (29). However, compared to conventional SCS, HD-SCS delivers more energy to the spinal cord and surrounding tissue. The long-term effects of such high electrical charge per time unit have not yet been evaluated. Second, HD-SCS consumes more energy from the SCS battery, which may affect the battery life of the neurostimulator. The purpose of this registry is not to compare the HD-SCS algorithm with other new developed subthreshold stimulation algorithms. We believe that the effectiveness of HD-SCS has to initially be established.
Despite the fact that HD stimulation overcomes known limitations of conventional SCS, such as uncomfortable paresthesia and increased pain awareness (7), proof of effectiveness and feasibility is scarce. This shortcoming can be perceived as a roadblock for its widespread implementation in clinical practice. The DISCOVER study will be the first and largest prospective, multicenter HD-SCS study conducted to date. The study results will provide insight into the clinical effectiveness and safety of HD SCS.