1. Background
Intravenous (I.V.) cannulation is a prerequisite before any major or minor procedure. It is an invasive approach wherein a catheter is introduced into the lumen of a peripheral vein through the patient’s skin (1). The pain and discomfort due to intravenous cannulation produce anxiety and stress for anyone and may lead to future negative ramifications like non-willingness for likely upcoming hospital admissions (2, 3). Several pharmacological and non-pharmacological methods have been tried. Eutectic Mixtures of Local Anesthetics (EMLA) cream is a device for pain reduction during I.V. cannulation. EMLA cream is a 5% oil and water-based admixture containing 25 mg/mL of lidocaine and 25 mg/mL of prilocaine. Lidocaine/prilocaine are both amide group-containing local anesthetic agents (4). EMLA cream is applied onto non-injured skin with an occlusive adhesive bandage. The local anesthetics accumulate near pain receptors of the skin, and the neuronal membranes are stabilized by inhibiting the ion flow. This prevents even the commencement of impulses and provides local anesthetic action.
Regardless of the benefits offered by EMLA cream, it has certain disadvantages, such as allergic dermatitis. It is also costly and has a prolonged duration for the onset of peak action. This reduces its feasibility to be used as a tool for pain reduction, especially in emergency situations (5). Ethyl-chloride as a cryoanalgesic spray prior to pediatric I.V. cannulation is a popular method. After applying any cryoanalgesic, the skin temperature falls by less than 10°C compared to the body temperature after a 10-second application (6, 7).
2. Objectives
The aim of this study was to identify a more advantageous and less time-consuming method for reducing pain prior to cannulation in adult groups.
3. Methods
This was designed to be an observational prospective cohort study which was conducted after obtaining approval from the Institutional Ethics Committee (IEC KMC MLR 08-19/345) and Clinical trial registry of India Number (CTRI/2020/07/026656) between the time period of December 2019 to July 2021. Patients who fulfilled the inclusion criteria and did not fulfill the exclusion criteria were included in this observational study by convenience, non-randomized sampling method. The inclusion criteria were all oriented American Society of Anesthesiologists (ASA) I, II, and III patients between the age of 18 and 75 years who could explain the nature and extent of pain posted for elective surgeries. Patients who were unwilling to participate in the study, with a history of allergy to local anesthetics, any hemodynamic instability, coagulopathy or bleeding diathesis or peripheral neuropathy or local skin infection, and failure in I.V. cannulation were excluded from the study. The study protocol was explained to the patients, and written informed consent was obtained from the patients. The patients were conscious during the procedures and were able to communicate appropriately about their pain experience. Monitors such as Electrocardiogram, Pulse oximetry, and non-invasive blood pressure in the preoperative area were connected. The study groups were sub-divided into two groups, each containing 70 participants: Group E: 1 mL EMLA cream (equivalent to 1 gram) was placed in a thick layer over a prominent vein on the dorsum of the hand with an occlusive dressing for 60 minutes, and I.V. cannulation with 18 G cannula was attempted and group V: Ethyl chloride spray was applied at a distance of 10 cm for 10 - 15 seconds. Liquid on the skin was allowed to evaporate, and I.V. cannulation with 18 G on the dorsum of the hand was attempted. Cannulation was performed after skin disinfection.
The Visual Analogue Scale (VAS) scores were recorded post-procedure using the hemodynamic parameters during the procedure. Movement/pulling away of hand on doing procedure was recorded, and cost analysis was done. With 95% confidence interval and 80% power, the sample size was calculated using the following formula:
Where Z1-α/2 = 1.96 (at 5% significance level with 95% confidence interval)
Z1 - β = 0.84 (with 80% power)
σ = 1.06 (standard deviation)
d = 5 (clinically significant difference)
n = 70 in each group
3.1. Statistical Analysis
Data were tabulated and charted in Microsoft Excel and analyzed using SPSS® software version 25 (IBM®). Descriptive statistics were performed. Frequency and percentage were calculated for all the qualitative variables involved in the study. Mean, median, interquartile range, and standard deviation (SD) were calculated for quantitative variables.
Mann-Whitney U test, unpaired t-test, Fisher’s exact test, and chi-square test were used to compare the two groups. Post hoc analysis was performed to compare the data across different time points by Bonferroni test. P-values < 0.05 were considered significant.
4. Results
It was observed that the groups were similar in terms of age, sex, and ASA physical status (Tables 1 and 2).
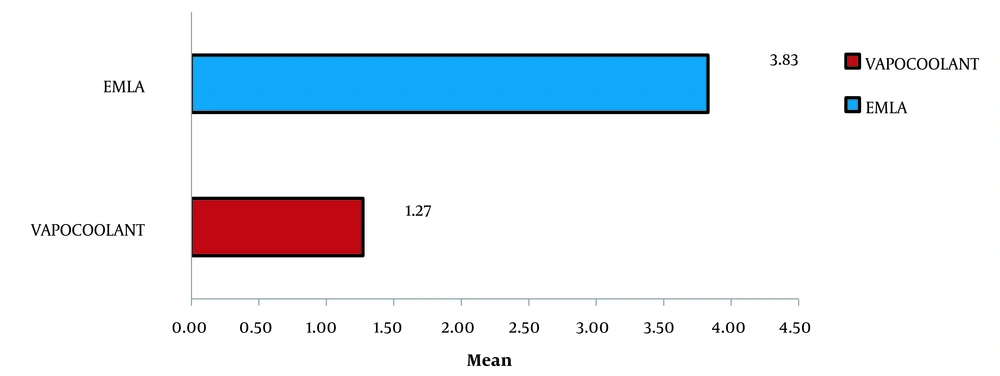
The mean VAS score for pain was 1.27 (1.191) in vapocoolant group and 3.83 (0.851) in the E-group, as seen in Table 3 and Figure 1.
| Groups (N = 70) | Mean ±SD | 50th (Median) | IQR | Mann-Whitney U Test, P-Value |
|---|---|---|---|---|
| Vapocoolant | 1.27 ± 1.191 | 1.00 | 0 - 2 | 0.0001, HS |
| EMLA | 3.83 ± 0.851 | 4.00 | 3 - 4.25 |
The Difference in VAS Scores in Vapocoolant Group vs. EMLA Cream Group Prior to I.V. Cannulation
Mann-Whitney U test was used to identify the variation in pain scores between the two groups, which was discerned as statistically significant (P-value = 0.0001). It was observed that the baseline heart rate was similar during I.V. cannulation in both groups: group V: 91.3 (14.4) beats min-1 and group E: 82.0 (12.2) beats min-1. However, an increase in heart rate was observed in both groups four minutes later. This increase in heart rate was higher in group E (105.6 (8.6) beats min-1) compared to group V (92.8 (15.3) beats min-1) (Tables 4 and 5).
| Parameter (HR) | N | Mean ± SD | 95% Confidence Interval for Mean | t-Test, P-Value a | |
|---|---|---|---|---|---|
| Lower Bound | Upper Bound | ||||
| Baseline | 0.001, HS | ||||
| Vapocoolant | 70 | 91.3 ± 14.4 | 87.9 | 94.7 | |
| EMLA | 70 | 82.0 ± 12.2 | 79.1 | 84.9 | |
| 2 min | 0.001, HS | ||||
| Vapocoolant | 70 | 94.5 ± 12.2 | 91.6 | 97.4 | |
| EMLA | 70 | 107.4 ± 6.0 | 106.0 | 108.9 | |
| 4 min | 0.001, HS | ||||
| Vapocoolant | 70 | 92.8 ± 15.3 | 89.1 | 96.5 | |
| EMLA | 70 | 105.6 ± 8.6 | 103.5 | 107.6 | |
| 8 min | 0.097, NS | ||||
| Vapocoolant | 70 | 93.6 ± 12.1 | 90.7 | 96.5 | |
| EMLA | 70 | 97.1 ± 12.4 | 94.1 | 100.0 | |
| 10 min | 0.787, NS | ||||
| Vapocoolant | 70 | 91.3 ± 13.3 | 88.2 | 94.5 | |
| EMLA | 70 | 90.7 ± 12.9 | 87.7 | 93.8 | |
| 15 min | 0.787, NS | ||||
| Vapocoolant | 70 | 86.1 ± 10.5 | 83.6 | 88.6 | |
| EMLA | 70 | 85.6 ± 10.7 | 83.0 | 88.2 | |
Heart Rate Variability Over 15 Minutes After I.V. Cannulation in Vapocoolant Spray Compared to EMLA Cream
| Parameter (MAP) | N | Mean ± SD | 95% Confidence Interval for Mean | t-Test, P-Value | |
|---|---|---|---|---|---|
| Lower Bound | Upper Bound | ||||
| Baseline | 0.907, NS | ||||
| Vapocoolant | 70 | 71.6 ± 6.5 | 70.1 | 73.1 | |
| EMLA | 70 | 71.7 ± 6.5 | 70.2 | 73.3 | |
| 2 min | 0.737, NS | ||||
| Vapocoolant | 70 | 72.0 ± 7.2 | 70.3 | 73.7 | |
| EMLA | 70 | 72.4 ± 7.8 | 70.5 | 74.3 | |
| 4 min | 0.851, NS | ||||
| Vapocoolant | 70 | 69.9 ± 6.0 | 68.4 | 71.3 | |
| EMLA | 70 | 70.1 ± 6.6 | 68.5 | 71.6 | |
| 8 min | 0.653, NS | ||||
| Vapocoolant | 70 | 71.8 ± 5.8 | 70.4 | 73.2 | |
| EMLA | 70 | 72.3 ± 6.6 | 70.7 | 73.9 | |
| 10 min | 0.537, NS | ||||
| Vapocoolant | 70 | 70.9 ± 4.8 | 69.7 | 72.0 | |
| EMLA | 70 | 71.4 ± 5.8 | 70.1 | 72.8 | |
| 15 min | 0.771, NS | ||||
| Vapocoolant | 70 | 73.5 ± 4.3 | 72.5 | 74.5 | |
| EMLA | 70 | 73.3 ± 4.3 | 72.2 | 74.3 | |
Mean Arterial Pressure (MAP) Variability in the Vapocoolant Group and EMLA Group
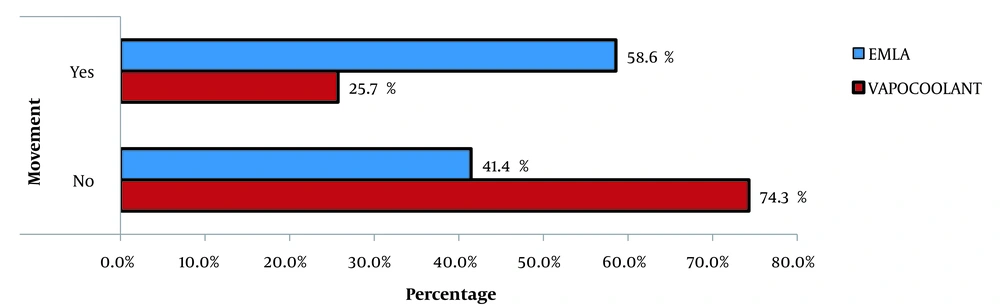
It was noted that there is no significant variation in other hemodynamic parameters, such as pulse oximetry and mean arterial pressure. Only 25.7 % of the participants in the vapocoolant group moved their hands during I.V. cannulation compared to 58.6% of the participants in the EMLA group (Figure 2).
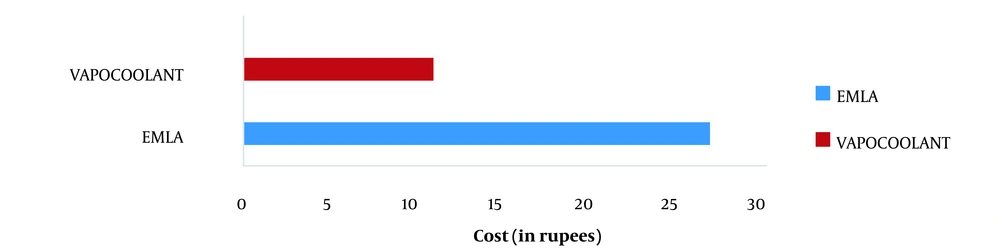
It was concluded that vapocoolant spray (Rs.11/-) was more cost-effective than EMLA cream (Rs. 27/-). A statistically significant difference was observed using Fisher's exact test (P-value = 0.001) (Figure 3).
5. Discussion
Intravenous (I.V.) cannulation is an essential practice during anesthesia, irrespective of any demographic factors. Most established non-invasive pharmacological modes of reducing discomfort due to affliction and fear in patients undergoing I.V. cannulation are onerous and tedious (like EMLA cream). This makes it to hinder some routine use, especially in emergency scenarios. However, ethyl chloride sprays provide momentary skin numbness within seconds of application. A recent randomized, controlled clinical trial was conducted with EMLA cream and vapocoolant spray with distraction techniques in children aged four to six years and those who were scheduled to receive diphtheria and tetanus toxoid vaccination during health supervision visits (8). It was concluded that the cry duration (in seconds) was 8.5 (21.0) vs. 38.6 (50.5) in those treated with vapocoolant spray vs. control. The VAS score in the vapocoolant group was 1.2 (1.9) compared to the control group, with a score of 3.1 (2.1). These values were similar and close to our study, wherein the VAS score was 1.27 (1.191) in patients on whom vapocoolant spray was used.
Another randomized, double-blind controlled trial on 80 pediatric patients aged between six-twelve years who received either vapocoolant spray or a placebo spray prior to I.V. cannulation showed a significant reduction in pain with the use of vapocoolant spray (VAS < 2 cm, 95% confidence interval [CI] 0 - 3 cm; P-value < 0.01) (9). First attempt cannulation was easier with the use of vapocoolant spray (85.0%) than with placebo (62.5%) (mean difference 22.5 %, 95%; P-value = 0.03). Similarly, in another study conducted on forty-one patients undergoing regular hemodialysis thrice weekly, it was shown that the pain intensity scoring based on VAS scoring with EMLA cream was significantly lower than that of vapocoolant spray (10). EMLA applications provided significantly lower total pain scores than all other interventions (P-value < 0.05). No patient experienced pain with EMLA cream (2 (1) cm) or vapocoolant spray (VAS: 2 (1) cm) compared to the controls (VAS: 3 (2) cm). It can be concluded from the similar VAS scores for both interventions that cryoanalgesic spray is as efficacious as EMLA cream. The patients reported VAS scores < 4 cm with EMLA cream and vapocoolant spray compared to control and placebo interventions. This contradictory result may be due to the fact that 25% of patients had diabetes mellitus as the etiology of renal failure. Hence, peripheral neuropathy cannot be ruled out in these patients.
According to a crossover randomized controlled trial on eighty pediatric patients with thalassemia who underwent I.V. cannulation for blood transfusion, vapocoolant spray was found to be inferior to EMLA cream in reducing VAS scores during I.V. cannulation (10). This could be attributed to the duration of the application of vapocoolant spray in their study. The vapocoolant spray was sprayed at a distance of 10 cm for 2 seconds. In our study, it was applied for 10 seconds. Therefore, our study showed that vapocoolant was more effective than EMLA cream with significantly lower VAS scores (1.27 (1.191)) (11).
Most patients in the EMLA cream group had a tingling/burning sensation after application compared to the cooling effect produced by vapocoolant spray. The sensation of cold was perceived by the adult as comfortable when compared to the burning sensation of EMLA cream. This could have been attributed to the reason why VAS scores were elevated in the EMLA group of patients. Anxiety due to the same can also lead to tachycardia, as is seen in this study. Nevertheless, the advantages of using vapocoolant spray due to its prompt action and cost-effectiveness in the adult population, which has not been studied, had to be impressed upon as per the results of the study.