1. Background
Hyperthermic intraperitoneal chemotherapy (HIPEC) following cytoreductive surgery (CRS) is a lengthy procedure, usually associated with considerable bleeding due to the large surgical field and the extensive nature of surgery (1). This results in both coagulation and pathophysiological changes, including an increase in the heart rate, body temperature, and metabolic acidosis. Raised intra-abdominal pressure may result in increased airway pressure and central venous pressure (2). The key role of the anesthesiologist is to anticipate, recognize, prevent, and manage these changes.
Minimizing the need for blood transfusions is one of the main goals in the perioperative management of patients undergoing HIPEC. In addition to the known adverse effects of blood transfusion, including postoperative infections, transfusion reaction, and economic burden (3, 4), blood transfusion is proven to be responsible for adverse outcomes in cancer surgery patients, including head and neck, ovarian, and lung cancer (5-7). It is associated with an increase in the cancer recurrence rate and a reduction in the total survival rate (8, 9).
In an attempt to decrease blood transfusion requirements, antifibrinolytic agents, including tranexamic acid (TA), are used. The main concern with their use was the fear of thromboembolic events, especially in cancer patients with an increased risk for thromboembolism (10). However, until now, there is no solid evidence that it increases the risk of thromboembolic events (11, 12).
A systematic review of various types of surgeries, including more than 10000 patients, showed that TA reduced transfusion rates by 38% (13).
2. Objectives
This study aimed to investigate whether the intraoperative administration of TA could reduce blood loss and transfusion requirements in patients undergoing CRS followed by HIPEC. Given the limited data on the safety and anti-hemorrhagic efficacy of TA in these types of surgeries, this study sought to address this knowledge gap. We also hypothesized that a single dose of TA given early after the induction of anesthesia would improve both blood loss and the need for blood transfusion.
3. Methods
After approval of the ethical committee at the National Cancer Institute, Cairo University (IRB number 201617027.2P), this study was prospectively registered at clinicaltrials.gov (NCT 03646474). Prior to participation in the study, all patients were provided with a thorough explanation of the procedures involved, and written informed consent was obtained from all patients. Sixty patients scheduled to undergo CRS followed by HIPEC were randomly assigned to 2 equal groups: Group 1 (TA group) received 10 mg/kg of TA in 100 mL of 0.9% NaCl over 20 minutes after the induction of anesthesia and before surgical incision; and group 2 (control group) that received a placebo of 100 mL of 0.9% NaCl during the same time interval.
Both ASA II and III patients scheduled for CRS followed by HIPEC, aged between 18 and 65 years, were included in the study. Patients with bleeding disorders and thrombophilia, as well as those with any past or current history of thromboembolic disease or a family history of such conditions, were excluded from the study. Allergy to TA, liver disease, or renal disease with a creatinine > 1.2 mg/dL, history of coronary stenting a year before surgery, cardiovascular problems, and patients on warfarin therapy for prophylaxis of thromboembolism were also excluded as well.
3.1. Perioperative and Surgical Procedure
Surgery was performed under general anesthesia and after routine preoperative evaluation. Anesthetic management was standardized for all patients. Before anesthesia, ceftriaxone (1 g) and midazolam (0.02 mg/kg) were administered as premedication. Induction of anesthesia was performed with 2 μg/kg fentanyl, 2 mg/kg propofol, and 0.5 mg/kg atracurium after pre-oxygenation with 100% oxygen. Immediately after the induction of anesthesia, an epidural catheter was inserted and fixed at the L2/L3 position with a 4- to 6-mL/h infusion of bupivacaine/fentanyl (0.125 mg/1 μg in 1 mL) mixture used for intraoperative analgesia. Anesthesia was maintained by administering 0.9 - 1.4 MAC of isoflurane and an intravenous infusion of atracurium at a rate of 0.3 to 0.6 mg/kg/h. End-tidal CO2 was maintained at 35 ± 5 mmHg. After the administration of 0.05 mg/kg of neostigmine and 0.01 mg/kg of atropine to reverse residual neuromuscular block, the patient was extubated fully awake and transferred to the postanesthesia care unit (PACU) for further monitoring and care.
Intravenous TA infusion over 20 minutes or an equivalent volume of 0.9% NaCl solution was given after induction and before surgical incision in the TA and control groups, respectively. A single postoperative daily dose of low-molecular-weight heparin (Clexane 1 mg/kg) was administered until patient discharge. Intraoperative fluids, whether crystalloids or colloids, were calculated. The blood transfusion trigger was a hemoglobin (Hb) level < 7 gm/L or a hemoglobin level < 8 gm/L in the presence of any physiological trigger (e.g., lactic acidosis, hypotension, tachycardia, and the presence of cardiovascular comorbidity or active blood loss). After 1 month, all patients were asked for a postoperative visit to be checked for any complications, including infection, stroke, myocardial infarction, pulmonary embolism, and renal failure, or any event that may be related to thromboembolism.
The primary endpoint was a reduction in total blood loss. However, the secondary endpoints were the total number of patients requiring transfusion and the rate of occurrence of postoperative thromboembolic events within a month after surgery.
The collected data were age, gender, body mass index (BMI), type and duration of surgery, and intraoperative mean arterial blood pressure. The levels of serum creatinine were measured preoperatively as well as on postoperative days 1, 3, and 5. The intraoperative and first 24-hour urine outputs were recorded. Hemoglobin levels were measured preoperatively (Hb pre, g/L), immediately after the operation, and on the first 5 days postoperatively (Hb post, g/L). To calculate intraoperative blood loss, the total blood in suction bottles was measured, and the blood in drapes and sponges was estimated. Additionally, the blood in drains during the first 24 hours was recorded.
The “hemoglobin balance method” was used to calculate the total losses (14). The patient’s blood volume was estimated using the lowest recorded hemoglobin level during the first 5 days after the surgery.
EBV (mL): The patient’s estimated blood volume before surgery.
Hbloss total (g): The loss volume of Hb.
Hbi (g/L): Preoperative Hb level.
Hbe (g/L): Postoperative Hb level.
Hbt (g): Total volume of blood transfusion.
Vloss total: Total RBC loss.
EBV calculation: Body wt (kg) × average blood volume (mL/kg)
A unit of banked blood typically contains 52 ± 5.4 g of Hb (15).
The number of patients requiring transfusions and the number of units of transfused red blood cells (RBCs) were documented. The patients were instructed to go to the emergency department if they experienced any symptoms of infection or thromboembolism.
3.2. Statistical Analysis
3.2.1. Sample Size Estimation
The sample size was estimated based on the study conducted by Seol et al. (16), where the mean ± SD in the control group was 886 ± 375.5 and 580 ± 655.0 in the tranexamic group. To achieve a 95% confidence level and a margin of error of 5%, the required sample size of 60 cases would be sufficient.
3.2.2. Data Management
Statistical analysis was performed using SPSS version 28 (IBM, Chicago, IL, USA). Quantitative parametric data were presented as mean and SD and were analyzed by unpaired Student’s t-test. Qualitative variables were presented as frequency and percentage and were analyzed by the chi-square test. Two-tailed P values less than 0.05 were considered statistically significant. The relative risk (with 95% CI) was measured to assess the probability of an event occurring with an exposure vs. the probability of the event occurring without the exposure.
4. Results
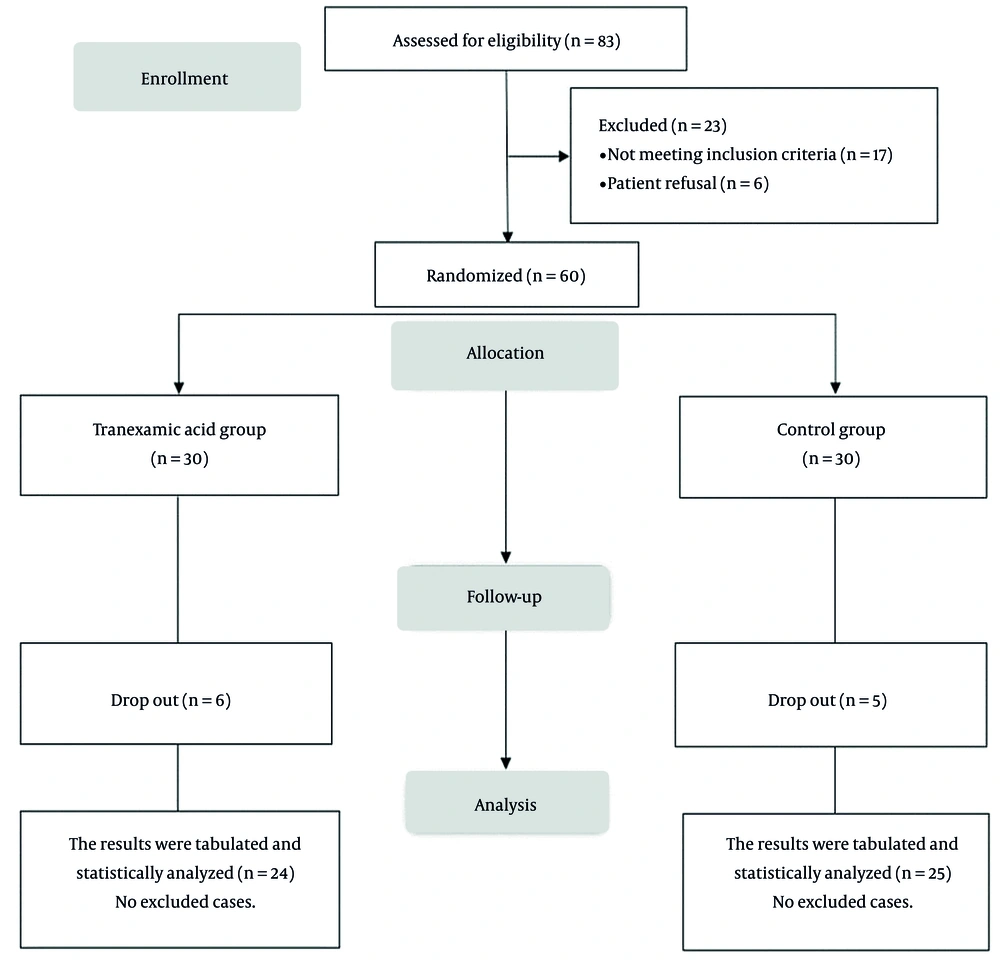
In this study, 83 patients were assessed for eligibility, 17 patients did not meet the criteria, and 6 patients refused to participate in the study. The remaining 60 patients were randomly allocated into 2 groups (30 patients in each group). Six patients from the TA group and 5 patients from the control group were excluded from the study due to various reasons, including massive blood loss (1 case in the TA group and 2 cases in the control group), prolonged surgery (2 cases in the TA group and 1 case in the control group), re-operation (2 cases in the TA group and 1 case in the control group), and being inoperable cases (1 case in the TA group and 1 case in the control group), see Figure 1.
There were no significant differences in patient characteristics, risk factors, or duration of surgery between the two groups (Table 1).
| Variables | TA Group (n = 24) | Control Group (n = 25) | Mean Difference or RR (95% CI) | P Value |
|---|---|---|---|---|
| Age (y) | 44.42 ± 11.02 | 43.4 ± 10.07 | 1.02 (-5.047 to 7.08) | 0.737 |
| ASA physical status | 0.694 (0.29 to 1.65) | 0.599 | ||
| II | 18 (75) | 16 (64) | ||
| III | 6 (25) | 9 (36) | ||
| BMI (kg/m2) | 18.16 ± 1.82 | 18.44 ± 2.01 | -0.27 (-1.377 to 0.83) | 0.620 |
| Risk factors | ||||
| Preoperative radiotherapy | 2 (8.33) | 8 (12.0) | 0.21 (0.05 to 0.92) | 0.635 |
| Preoperative chemotherapy | 11 (45.83) | 14 (56.0) | 0.82 (0.47 to 1.43) | 0.455 |
| DM | 12 (50.0) | 11 (44.0) | 1.14 (0.62 to 2.06) | 0.798 |
| Hypertension | 10 (41.67) | 13 (52.0) | 0.80 (0.43 to 1.46) | 0.447 |
| IHD | 13 (54.17) | 14 (56.0) | 0.96 (0.58 to 1.60) | 0.809 |
| Duration of surgery (h) | 4.98 ± 0.83 | 5.19 ± 0.64 | -0.21 (-0.639 to 0.213) | 0.320 |
Demographic Data and Duration of Surgery in the Studied Groups a
No significant differences were found in Hb measurements (immediately before and after surgery and on postoperative days 1, 2, 3, 4, and 5), serum creatinine (on postoperative days 1, 2, and 5), or urine output (during the surgery and within the first 24 hours after surgery) between the 2 groups (Table 2, Figures 2 and 3).
| Variables | TA Group (n = 24) | Control Group (n = 25) | Mean Difference (95% CI) | P Value b |
|---|---|---|---|---|
| Hb (gm/dL) | ||||
| Immediate preoperative | 11.11 ± 0.85 | 11.09 ± 0.79 | 0.02 (-0.45 to 0.49) | 0.932 |
| Immediate postoperative | 10.16 ± 0.8 | 9.76 ± 0.84 | 0.39 (-0.07 to 0.86) | 0.097 |
| First day postoperative | 10.2 ± 0.68 | 9.76 ± 1.02 | 0.44 (-0.06 to 0.93) | 0.086 |
| Second day postoperative | 10.4 ± 0.68 | 9.9 ± 1.11 | 0.50 (-0.03: 1.03) | 0.064 |
| Third day postoperative | 10.9 ± 0.7 | 10.67 ± 0.75 | 0.24 (-0.18 to 0.65) | 0.262 |
| Fourth day postoperative | 10.97 ± 0.74 | 10.89 ± 0.78 | 0.08 (-0.35 to 0.51) | 0.718 |
| Fifth day postoperative | 11.02 ± 0.79 | 11 ± 0.79 | 0.02 (-0.43 to 0.47) | 0.942 |
| Serum creatinine (mg/dL) | ||||
| Preoperative | 0.85 ± 0.16 | 0.86 ± 0.16 | -0.01 (-0.105 to 0.076) | 0.750 |
| First day postoperative | 1.15 ± 0.2 | 1.23 ± 0.24 | -0.08 (-0.204 to 0.052) | 0.237 |
| Third day postoperative | 1.03 ± 0.15 | 1.16 ± 0.32 | -0.13 (-0.273 to 0.013) | 0.074 |
| Fifth day postoperative | 0.99 ± 0.13 | 1.02 ± 0.19 | -0.03 (-0.127 to 0.062) | 0.491 |
| Urine output (mL) | ||||
| Intraoperative | 1052.04 ± 230.08 | 1153.72 ± 246.74 | -101.68 (-238.93 to 35.57) | 0.143 |
| First 24 hours postoperative | 1862.08 ± 266.65 | 1911.6 ± 270.32 | -49.52 (-203.90 to 104.86) | 0.522 |
Hemoglobin, Serum Creatinine, and Urine Output Within the First 24 Hours After Surgery a
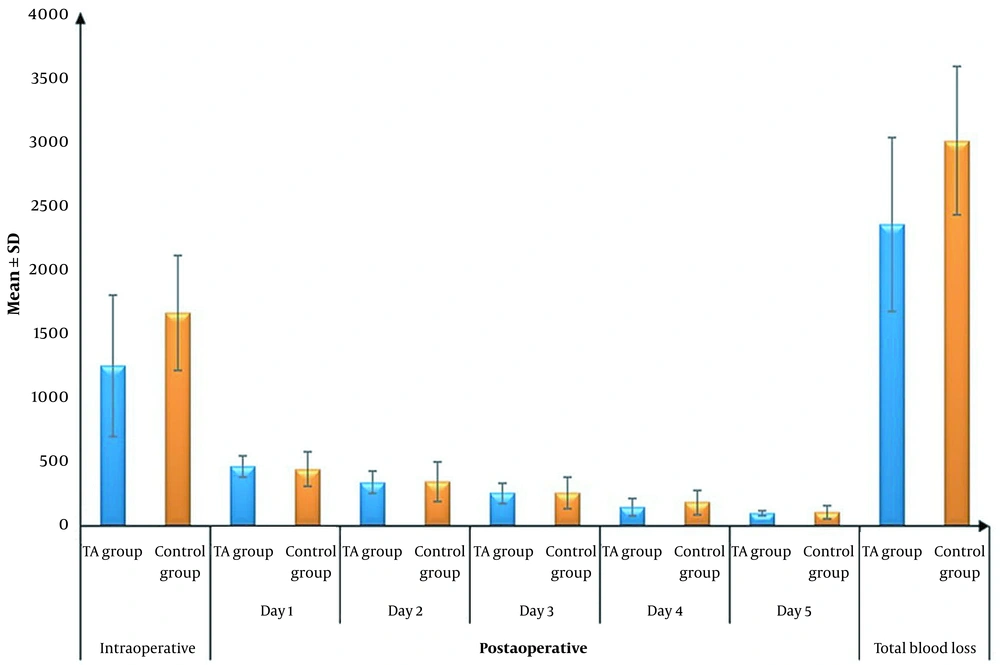
The mean ± SD intraoperative and postoperative blood loss on the first day and total blood loss was significantly lower in the TA group than in the control group (1245.83 ± 550.87 vs. 1656 ± 448.22, 465 ± 85.45 vs. 443.6 ± 131.87, and 2346.25 ± 674.07 vs. 2997.8 ± 575.54; P = 006, 0.035, and 0.001, respectively). There were no significant differences in postoperative blood loss on days 2, 3, 4, and 5 between the 2 groups (Table 3 and Figure 4).
| Variables | TA Group (n = 24) | Control Group (n = 25) | Mean Difference (95% CI) | P Value |
|---|---|---|---|---|
| Intraoperative blood loss (mL) | 1245.83 ± 550.87 | 1656 ± 448.22 | -410.17 (-699.92 to -120.41) | 0.006 b |
| Postoperative blood loss (mL) | ||||
| First day | 465 ± 85.45 | 443.6 ± 131.87 | -69.02 (-132.83 to -5.20) | 0.035 b |
| Second day | 340 ± 87.72 | 345.6 ± 150.78 | -58.93 (-130.46 to 12.59) | 0.104 |
| Third day | 255 ± 75.95 | 258.8 ± 125.58 | -58.18 (-118.30 to 1.95) | 0.058 |
| Fourth day | 145 ± 66.65 | 184.8 ± 95.49 | -35.63 (-83.47 to 12.20) | 0.141 |
| Fifth day | 100 ± 19.5 | 109 ± 50.7 | -19.63 (-41.92 to 2.67) | 0.083 |
| Total blood loss (mL) | 2346.25 ± 674.07 | 2997.8 ± 575.54 | -651.55 (-1012.82 to -290.28) | 0.001 b |
Intraoperative, Postoperative, and Total Blood Loss in the Studied Groups a
Fluid replacement therapy and the need for intraoperative blood transfusion were both significantly lower in the TA group than in the control group (P = 0.026 and 0.032, respectively). The number needed to treat (NNT) was 3.352 (95% CI, 1.84 - 18.49). The numbers of blood and plasma units (intraoperative and postoperative) were significantly lower in the TA group than in the control group (P < 0.05; Table 4).
| Variables | TA Group (n = 24) | Control Group (n = 25) | Mean Difference or RR (95% CI) | P Value |
|---|---|---|---|---|
| Fluid replacement therapy | 4545.83 ± 763.85 | 5004 ± 625.49 | - 458.17 (-860.92 to -55.42) | 0.026 b |
| Blood transfusion | ||||
| Intraoperative | 13 (54.17) | 21 (84.0) | 0.64 (0.43 to 0.97) | 0.032 b |
| Postoperative till the fifth day | 8 (33.33) | 10 (40.0) | - | 0.695 |
| Number of blood units | ||||
| Intraoperative | 1.58 ± 1.74 | 2.88 ± 1.48 | - 1.29 (-2.23 to -0.364) | 0.007 b |
| Postoperative | 0.46 ± 0.72 | 1.24 ± 1.67 | 0.039 ( -1.52 to -0.041) | 0.040 b |
| Number of Plasma units | ||||
| Intraoperative | 0.92 ± 1.35 | 1.8 ± 1.44 | - 883 (-1.69 to -0.081) | 0.032 b |
| Postoperative | 0.13 ± 0.34 | 0.84 ± 1.21 | -0.715 ( -1.23 to -0.198) | 0. 008 b |
Blood Transfusion and the Number of Blood and Plasma Units in the Studied Groups a
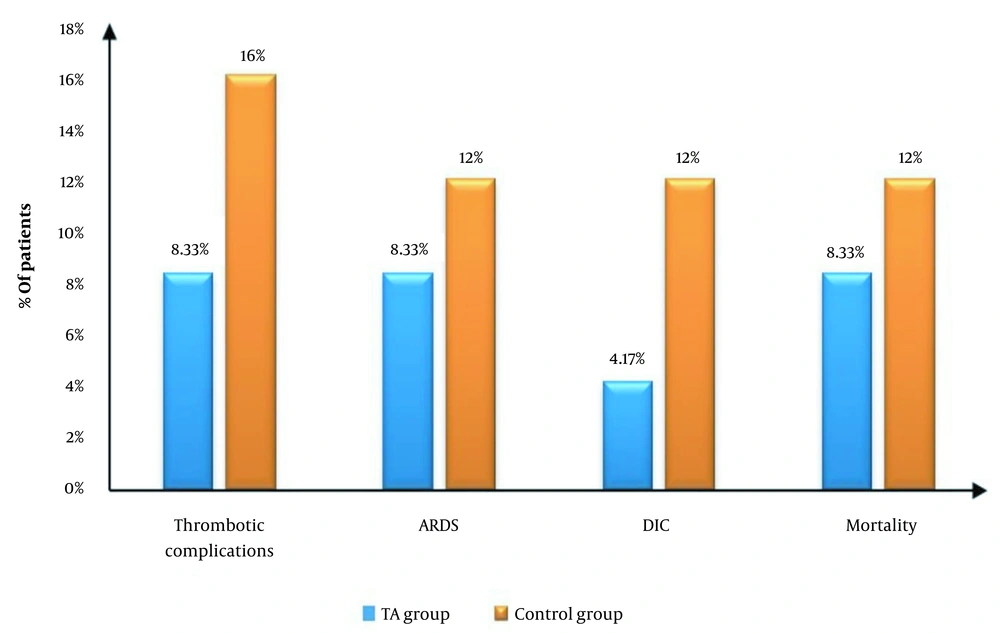
Thrombotic complications occurred in 2 (8.33%) patients in the TA group and 4 (16%) patients in the control group. Acute respiratory distress syndrome (ARDS) occurred in 2 (8.33%) patients in the TA group and 3 (12.0%) patients in the control group. Disseminated intravascular coagulation (DIC) occurred in 1 (4.17%) patient in the TA group and 3 (12.0%) patients in the control group. Mortality occurred in 2 (8.33%) patients in the TA group and 3 (12.0%) patients in the control group. There were no statistically significant differences in the incidence of complications between the 2 studied groups (Table 5 and Figure 5).
| Variables | TA Group (n = 24) | Control Group (n = 25) | RR (95% CI) | P Value |
|---|---|---|---|---|
| Thrombotic complications | 2 (8.33) | 4 (16) | 0.52 (0.10 to 2.59) | 0.667 |
| ARDS | 2 (8.33) | 3 (12.00) | 0.69 (0.13 to 3.79) | 1 |
| DIC | 1 (4.17) | 3 (12.00) | 0.34 (0.04 to 3.1) | 1 |
| Mortality | 2 (8.33) | 3 (12.0) | 0.69 (0.13 to 3.79) | 1 |
The Incidence of Complications in the Studied Groups a
5. Discussion
Extensive cancer surgery is frequently associated with significant blood loss and the use of allogeneic blood transfusion (17, 18). Many studies have shown the detrimental effects of allogeneic blood transfusion on patients’ cancer outcomes (19, 20). One of the major cancer surgeries is CRS with HIPEC. This procedure offers a promising treatment modality for many peritoneal malignancies, including colorectal, gastric, and ovarian cancer, as well as pseudomyxoma peritonei and peritoneal mesothelioma (21-23). Despite the promising results, this combined modality has a high rate of complications (24). It is a lengthy procedure that involves extensive exploration and resection, with a large surgical field, and often requires blood transfusion (24, 25).
In the current study, the authors found that a single dose of TA given after induction and before surgical incision significantly decreased the incidence of both blood loss and transfusion rates in HIPEC without any additional increased risk of postoperative thromboembolic events.
In agreement with this study, a systematic review, including 723 patients who received TA and 659 control patients who underwent elective abdominal or pelvic cancer surgeries, showed a reduction in blood loss and transfusion rates without any remarkable increase in postoperative venous thromboembolism (VTE) with TA use (25).
In line with the current study, Oyama et al. showed that TA reduced blood loss after bone and soft tissue tumors with wide resection surgeries (26). They reported no thromboembolic complications in any group of patients (26).
A retrospective review of 104 orthopedic cancer surgeries showed that antifibrinolytics (such as TA) resulted in no increased risk of thromboembolic complications. Physicians avoided routine use of TA in cancer patients, as cancer is known to cause a hypercoagulable state. However, emerging evidence suggests that the use of TA may be considered safe in cancer patients, contrary to previous beliefs (27).
In a meta-analysis conducted by Koh et al., involving 429 patients from 6 studies, it was found that TA reduced the perioperative blood transfusion requirement in hepatic resection and transplantation without an increase in the incidence of thromboembolic events (28).
Furthermore, in patients undergoing resection of colorectal liver metastases, Jaffer et al. showed that intraoperative TA resulted in a reduction of blood transfusion for 30 days postoperatively without any reported increase in thromboembolic events (29). In a meta-analysis conducted by Koh et al., which included 19 studies on 2205 patients who underwent extra-hepatic abdominal surgery, TA use resulted in a significant reduction in intraoperative blood loss and perioperative blood transfusion without an increase in the incidence of thromboembolic events (30).
In accordance with the current study, another meta-analysis (including a variety of surgical procedures) showed a reduction in perioperative blood loss and transfusion rates without any reported increase in thromboembolic events after a single dose of intravenous TA given preoperatively. Furthermore, this meta-analysis recommends that TA be used prophylactically in surgery (31).
In contrast to this study, Shiralkar et al. did not report any significant difference in blood loss between patients who received TA and those who did not receive TA during the perioperative management of pseudomyxoma peritonei by CRS (24). This can be explained by the fact that it was a retrospective audit where patients underwent either CRS alone or CRS with HIPEC or CRS with HIPEC and early postoperative intraperitoneal chemotherapy with only 42.9% of all patients received TA irrespective of the type of the procedure. Moreover, they concluded that the deep venous thrombosis (DVT) incidence was much higher in patients receiving TA. However, the study was retrospective, and many preoperative comorbidities can be involved in this finding (24).
Wright et al. also conducted a randomized study of preoperative TA vs. placebo in major oncologic operations, including HIPEC, and concluded that TA did not decrease blood transfusion rates or blood loss (32). However, they decided to stop their study early and accept the null hypothesis based on a low conditional probability of achieving a positive result. In agreement with the current study, they showed no evidence of increased thromboembolic events with TA. These contradicting results may be attributed to different types of surgeries included in their study; each surgery may be associated with a different pathophysiology for blood loss (32).
A meta-analysis of all randomized clinical trials examining the effect of TA on cancer patients undergoing head and neck surgery demonstrated that TA reduced postoperative bleeding, which is consistent with the findings of the current study that showed a reduction in bleeding on the first postoperative day. They also found no difference in postoperative Hb levels. However, unlike the current study, they found no difference in intraoperative blood loss. This can be explained by the different regimens used for TA administration in each randomized clinical trial, which may affect the incidence of blood loss (33).
5.1. Conclusions
In this study, despite extensive blood loss associated with CRS/HIPEC procedures, TA proved to be a safe and effective method for reducing perioperative blood loss and transfusion requirements with no adverse effects. The relatively small sample size and the short follow-up interval for monitoring the occurrence of thromboembolic complications are limitations of this study.