1. Background
Patients with lung disease may exhibit pathological pulmonary ultrasound findings, including irregularities in the pleural line, pleural effusion, atelectasis, pulmonary consolidations, and an increasing number of B-lines that correlate with severity (1, 2). It is worth noting that these pathological findings can also be observed in patients who have had previous respiratory infections and subsequently develop varying degrees of interstitial disease over time (3, 4).
B-lines are distinct laser-like vertical hyperechoic lines originating from the pleural plane. They extend uninterrupted to the bottom of the ultrasound screen and move in sync with lung sliding (5). The presence of three or more B-lines between two ribs indicates the presence of ultrasonographic interstitial syndrome, which can manifest as focal, multifocal, homogeneous, or diffuse patterns. However, the specificity of the ultrasonographic interstitial syndrome is limited and does not provide a definitive classification of the underlying diseases associated with the presence of B-lines (6). Additionally, ultrasonographic pulmonary abnormalities have been observed even in healthy individuals (7). Pathological ultrasonographic findings may suggest potential lung damage, resulting in reduced static compliance of the lung parenchyma (8-10). Consequently, low-compliance lungs are characterized by stiffness, requiring significantly higher pressures to achieve a given volume compared to high-compliance lungs.
Currently, the prevalence of pulmonary ultrasound abnormalities in patients without a history of lung disease and their potential correlation with lung compliance remains undisclosed.
2. Objectives
The primary aim of this study is to investigate ultrasound-detected preoperative pulmonary abnormalities in patients without any documented respiratory conditions and evaluate their relationship with underlying pathologies and static lung compliance.
3. Methods
This single-center prospective observational study received approval from the Ethics Committee at Vall d'Hebron University Hospital (PR(AG)346/2020, date: June 26, 2020). Written informed consent was obtained from all participants prior to their inclusion in the trial. The trial was retrospectively registered on clinicaltrials.gov (NCT04922931).
3.1. Eligibility Criteria and Patient Selection
This study enrolled consecutive patients aged ≥ 18 years who underwent elective surgery between February 18, 2021, and March 5, 2021. The surgical specialties included urology, general surgery, otolaryngology, ophthalmology, plastic surgery, gynecology, neurology, cardiac surgery, thoracic surgery (specifically mediastinoscopy and sympathectomy), as well as various anesthetic techniques such as local anesthesia, general anesthesia, neuraxial anesthesia, and peripheral regional anesthesia.
This cohort consisted of patients who had no previous history of SARS-CoV-2 infection and tested negative for SARS-CoV-2 through pre-operative screening using PCR. Furthermore, these patients did not exhibit any underlying pulmonary pathology at baseline.
The following exclusion criteria were applied: Patients below the age of 18, pregnant individuals, patients with a mean arterial pressure < 60 mmHg requiring vasopressors, patients with existing pulmonary hypertension or heart failure, those who have undergone or are scheduled for pulmonary lobectomy or pleural surgery, individuals with pre-existing pulmonary pathology (obstructive or restrictive) prior to surgery, and patients currently experiencing respiratory infections.
3.2. Lung Ultrasound and Pulmonary Compliance
Pulmonary alterations were assessed using a convex probe in combination with the Sonosite portable ultrasound system and a 2- to 5-MHz convex transducer. For the evaluation of pleural irregularities, higher frequencies (11-13) were employed, and a linear probe with a frequency of 6 - 15 MHz was used if required. A total of ten thoracic areas were examined, with five areas in each hemithorax. The first four areas in each hemithorax corresponded to the exploration conducted by Volpicelli et al. (14), and an additional fifth area was included to assess the posterior region of the thorax at the level of the posterior axillary line. Areas 1 and 2 were defined as the upper anterior and lower anterior thoracic regions, respectively. Areas 3 and 4 corresponded to the upper lateral and basal lateral thoracic regions, respectively. During supine decubitus, the anterolateral areas were delimited by three longitudinal lines (parasternal, anterior, and posterior axillary), while a breast line defined the upper and lower zones of each hemithorax. This configuration resulted in five specific exploration points: (1) middle-clavicular line at the 2 - 3 intercostal spaces, (2) middle-clavicular line at the 5 - 6 intercostal spaces, (3) half-axillary line at the 2 - 3 intercostal spaces, (4) half-axillary line at the 5 - 6 intercostal spaces, and (5) posterior axillary line at the 5 - 7 intercostal spaces. The probe was positioned perpendicular to the ribs at a depth of 9 - 12 cm anteriorly and 12 - 20 cm laterally to the hemithorax, focusing on the pleural level. Each region was scored based on the following pulmonary ultrasound features:
- Zero points: Presence of lung sliding with A lines or one or two isolated B-lines.
- One point: Three or more B-lines with a noticeable gap indicating moderate loss of lung aeration.
- Two points: Extensive area of crowded and merging B-lines suggesting severe loss of lung aeration.
- Three points: Presence of hypoechoic, poorly-defined tissue signifying complete loss of lung aeration.
The lung ultrasound score, ranging from 0 to 30, was obtained by summing the points assigned in each region.
All patients underwent neuromuscular blockade during surgery and were subjected to volume-controlled ventilation with a tidal volume of 7 mL/kg based on ideal body weight, along with positive end-expiratory pressure (PEEP) of 5 mmHg. Static compliance was calculated in the supine decubitus position using the following formula: Static compliance = tidal volume/plateau pressure - PEEP.
3.3. Data Collection
Baseline variables documented included age, gender, American Society of Anesthesiologists (ASA), physical status classification, and underlying pathologies. Lung ultrasound data collection involved noting the number of B-lines in each lung area, as well as the presence of atelectasis, pleural effusion, or pleural irregularities in specific lung fields. Operative variables encompassed the type of anesthesia administered, the surgical procedure performed, the anesthetic technique utilized, and the requirement for transfusions. Additionally, the surgical risk was assessed based on the classification provided by the National Institute for Clinical Excellence (NICE) of the UK's National Health Service, and this information was also recorded.
Postoperative data collection encompassed 30-day survival following surgery and the occurrence of PPC during the hospital stay. The specific PPCs considered were as follows:
1. Respiratory infection: Defined by a white blood cell count > 12,000/µL or the presence of pulmonary opacities or fever.
2. Acute respiratory distress syndrome: Diagnosed based on the presence of bilateral pulmonary infiltrates and an arterial partial pressure of oxygen to fraction of inspired oxygen (PaO2/FiO2) ratio of less than 200.
3. Atelectasis: Identified by pulmonary opacification along with displacement of the affected area's hemidiaphragm, hilum, or mediastinum.
4. Bronchospasm: Indicated by wheezing that required bronchodilator therapy.
5. Pneumothorax: Noted in the presence of air within the pleural cavity.
6. Pulmonary thromboembolism: Diagnosed using chest computed tomography angiography in combination with the symptom of dyspnea.
3.4. Statistical Analysis
Categorical variables were presented as frequencies and percentages, while continuous variables were expressed as mean and standard deviation. The Spearman correlation coefficient was utilized to assess the correlation between two variables. All statistical analyses were performed using IBM SPSS Statistics 25.
4. Results
One hundred and four patients, who did not have diagnosed lung disease or associated respiratory symptoms, were included in the study. The average age of the patients was 59.46 ± 16.06 years. A total of 14 patients exhibited lung ultrasound abnormalities. None of the patients displayed subpleural consolidations. Among the patients, five (4.8%) presented with atelectasis, while four (3.8%) had pleural effusion.
Twenty-four (23.07%) patients presented 1 - 2 B-lines in some lung field. Ultrasound B-lines score was > 0 (≥ 3 B-lines) in seven patients (6.7%). Among these patients, the mean number of lung fields with ≥ 3 B-lines was 3.71 ± 2.43. Five (71.42%) patients with ≥ 3 B-lines showed bilateral distribution and also presented pleural abnormalities. Only two patients presented a lung field with B-lines ≥ 3.
Out of the total patients, ten (9.6%) exhibited pleural irregularities. Among these, five patients had ≥ 3 B-lines, one patient had pleural effusion, and two patients had atelectasis. Atelectasis was observed independently in two patients.
Table 1 displays the B-lines score and lung static compliance. The average value of lung compliance was 56.78 ± 15.33. Among the fourteen patients with ultrasound abnormalities, eleven of them underwent general anesthesia, with recorded pulmonary compliance values.
| Parameters | No. (%) |
|---|---|
| Pleural irregularities | |
| Total | 104 (100) |
| No | 94 (90.4) |
| Yes | 10 (9.6) |
| B-lines score | |
| Total | 104 (100) |
| 0 | 97 (93.3) |
| 1 | 1 (1) |
| 2 | 2 (1.9) |
| 3 | 1 (1) |
| 4 | 1 (1) |
| 5 | 0 (0) |
| 6 | 0 (0) |
| 7 | 2 (1.9) |
| Compliance mL/cm H2O | |
| Total | 72 (100) |
| < 40 | 5 (6.9) |
| 40 - 49 | 15 (20.8) |
| 50 - 59 | 27 (37.5) |
| 60 - 70 | 13 (18.1) |
| > 70 | 12 (16.7) |
The pathological findings identified through lung ultrasound did not show any association with age, sex, or pre-existing baseline pathologies. However, patients classified as ASA ≥ II exhibited a higher prevalence of B-lines compared to patients classified as ASA I (Table 2).
| Total (n = 104) | Pleural Irregularities (n = 10) | B-Lines (n = 7) | Compliance | |||||
|---|---|---|---|---|---|---|---|---|
| No. (%) | P-Value | No. (%) | P-Value | No. (%) (n = 72) | No. (%), < 50 (n = 20) | P-Value | ||
| Age | ||||||||
| < 50 | 31 (29.8) | 2 (20) | 0.65 | 2 (28.6) | 0.95 | 23 (31.9) | 6 (30) | 0.79 |
| 50 - 69 | 39 (37.5) | 5 (50) | 3 (42.9) | 28 (38.9) | 7 (35) | |||
| ≥ 70 | 34 (32.7) | 3 (30) | 2 (28.6) | 21 (29.2) | 7 (35) | |||
| Sex | ||||||||
| Woman | 49 (47.1) | 3 (30) | 0.25 | 3 (42.9) | 0.81 | 36 (50) | 10 (50) | 1 |
| Man | 55 (52.9) | 7 (70) | 4 (57.1) | 36 (50) | 10 (50) | |||
| ASA | ||||||||
| I | 11 (10.6) | 1 (10) | 0.91 | 0 (0) | 0.039 | 8 (11) | 2 (10) | 0.81 |
| II | 53 (51) | 6 (60) | 5 (71.4) | 36 (50) | 11 (55) | |||
| III | 38 (36.5) | 3 (30) | 1 (14.3) | 26 (36.1) | 7 (35) | |||
| IV | 2 (1.9) | 0 (0) | 1 (14.3) | 2 (2.8) | 0 (0) | |||
| Cardiopathy | ||||||||
| Valvular | 22 (22.1) | 3 (30) | 0.51 | 2 (28.6) | 0.75 | 13 (18.1) | 4 (20) | 0.44 |
| Ischemic | 5 (4.8) | 1 (10) | 0 (0) | 4 (5.6) | 0 (0) | |||
| Vasculopathy | 15 (14.4) | 1 (10) | 0.17 | 2 (28.6) | 0.27 | 10 (13.9) | 1 (5) | 0.17 |
| Renal disease | 16 (15.4) | 1 (10) | 0.24 | 2 (28.6) | 0.31 | 10 (13.9) | 2 (10) | 0.55 |
| Hypertension | 50 (48.1) | 4 (40) | 0.28 | 4 (57.1) | 0.61 | 33 (45.8) | 11 (55) | 0.33 |
| Diabetes | 21 (20.2) | 2 (20) | 0.98 | 1 (14.3) | 0.68 | 12 (16.7) | 4 (20) | 0.63 |
Abbreviation: ASA, American Society of Anesthesiologists.
a Data are expressed as frequency (%).
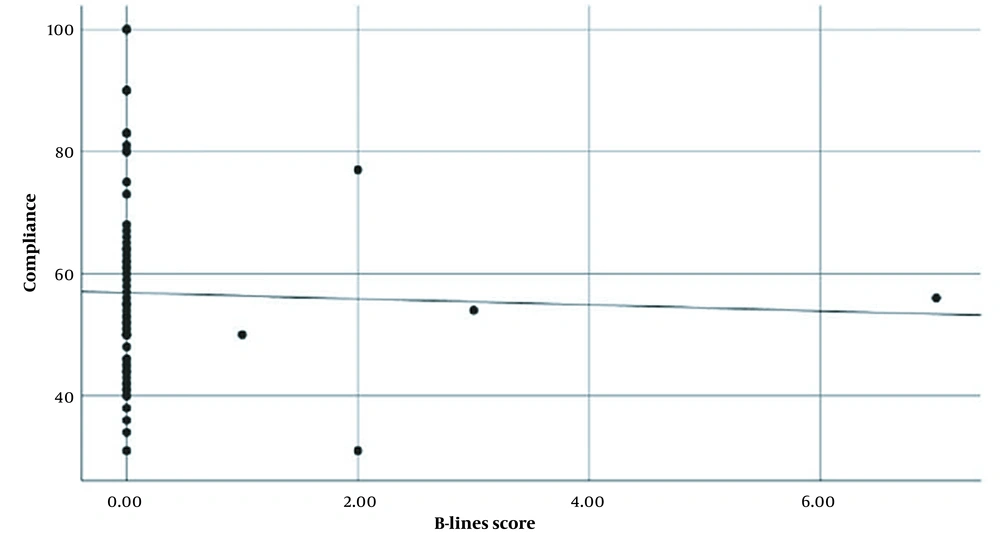
No significant correlation was observed between the overall score of the B-lines and lung compliance, as indicated by Spearman's correlation (rho = -0.028, P-value = 0.812), as depicted in Figure 1. The surgical characteristics are presented in Table 3.
| Parameters | n = 104, No. (%) |
|---|---|
| Surgery | |
| General | 46 (44.2) |
| Urology | 19 (18.3) |
| Vascular | 9 (8.7) |
| Neurology | 8 (7.7) |
| Cardiac | 9 (8.7) |
| Thoracic | 3 (2.9) |
| Otorhinolaryngology | 4 (3.8) |
| Gynecology | 3 (2.9) |
| Ophthalmology | 1 (1) |
| Plastic | 2 (1.9) |
| Surgical complexity | |
| I | 19 (18.3) |
| II | 36 (34.6) |
| III | 35 (33-7) |
| IV | 14 (13.5) |
| Type of anesthesia | |
| General | 78 (75) |
| Spinal | 21 (20.19) |
| Peripheral | 0 (0) |
| Local | 5 (4.80) |
Three patients (2.9%) experienced PPC. Among them, one patient required non-invasive mechanical ventilation due to acute respiratory distress syndrome, another patient developed pneumonia, and one patient had atelectasis necessitating non-invasive mechanical ventilation. Additionally, thirteen patients (12.5%) encountered non-respiratory pulmonary complications. Fortunately, all patients survived beyond the 30-day postoperative period.
5. Discussion
Our findings revealed that asymptomatic patients without baseline pulmonary pathology may exhibit pulmonary ultrasound abnormalities such as pleural abnormalities and pathological B-lines. In addition, patients with ASA ≥ II demonstrated a higher prevalence of pulmonary B-lines than patients with ASA I.
In our study, we identified B-lines ≥ 3 in seven (6.73%) patients, all of whom had underlying systemic diseases. The ASA physical status classification system is a valuable tool utilized to evaluate and document a patient's pre-anesthetic medical comorbidities (15-17). While the classification system alone does not independently predict perioperative risks, when combined with other factors such as the type and severity of surgery, as well as frailty, it can be helpful in predicting perioperative risks (15-17). This classification system employs a scale ranging from I to VI, taking into account the patient's medical history, current physical condition, and the severity of known medical conditions. Grade I represents a healthy patient with minimal risks, while grade VI corresponds to a brain-dead patient with plans for organ donation. In our study, ASA I patients did not exhibit any pathological B-lines; however, patients classified as ASA II (mild systemic disease), ASA III (severe systemic disease), or ASA IV (serious health-threatening systemic disease) displayed ≥ 3 B-lines. There is a lack of studies investigating the predictive value of the ASA score for abnormalities observed in lung ultrasound. Systemic processes can affect the airways, pulmonary parenchyma, vasculature, pleural membranes, and respiratory muscles to varying extents.
In our study, 24 (23.07%) patients classified as ASA I exhibited 1-2 B-lines in certain lung fields. Zoneff et al. (7) previously reported the presence of B-lines in healthy volunteers without respiratory disease symptoms; however, the "normal" number of B-lines remains unknown. It is believed that the presence of B-lines in the lateral and basal areas of each hemithorax may be considered normal, and approximately 34% of hospitalized patients may have ≥ 3 B-lines (18). Additionally, Raiteri et al. (19) discovered that lung ultrasound abnormalities were not uncommon in healthy individuals, with 26% of subjects exhibiting such abnormalities.
Among the seven patients in our study with pathological B-lines, five exhibited diffuse B-lines in multiple lung areas, suggestive of interstitial edema. In contrast, only two female patients displayed isolated pathological B-lines in the right lateral and posterior lung fields. These particular B-lines could potentially be attributed to the presence of the right horizontal cleft lung (7).
Pleural irregularities, characterized by the absence of the normal hyperechogenic linear pleural contour (12, 20), were observed in 10 patients during our study. The exact significance of these irregularities remains unclear; however, in five patients, their presence correlated with the occurrence of B-lines, potentially indicating subpleural interstitial alterations with reduced lung aeration in the peripheral regions (21). Changes in the pleural line and the presence of multiple B-lines are commonly observed in early stages of pulmonary edema, as well as in other interstitial lung diseases like emphysema or pulmonary fibrosis (22, 23).
In our study, pleural effusion was observed in four patients (3.8%). Among these cases, three patients presented mild to moderate bilateral effusions, suggestive of a likely cardiogenic and/or renal etiology. In one patient, a unilateral pleural effusion of uncertain origin was detected, with the medical history revealing only arterial hypertension.
Despite atelectasis being the most common postoperative pulmonary complication following general anesthesia (24), our study revealed that five patients (4.8%) already had atelectasis prior to the initiation of surgery. The presence of atelectasis can negatively impact gas exchange, potentially resulting in hypoxemia and increased hospital mortality and length of stay in the intensive care unit (25).
We observed no significant correlation between lung static compliance and the score of the B-lines in our study. However, it is worth noting that such a correlation was observed in patients who had previously experienced a respiratory infection (3). The majority of patients in our study exhibited a B-lines score of zero (≤ 3 B-lines), and the number of patients with pathological B-lines (≥ 3 B-lines) and available records of static compliance under general anesthesia was quite limited. Moreover, the recorded lung compliance values exhibited considerable variation. Reduced compliance during anesthesia is believed to be a consequence of decreased functional residual capacity and the development of atelectasis. In intubated patients without pre-existing lung disease, the normal range for lung compliance is typically 50 to 70 mL/cm H2O. However, it can be reduced by certain medical conditions such as fibrosis, pulmonary hypertension, and congestion, or increased by conditions like asthma and pulmonary emphysema (26). It is important to note that these possibilities were ruled out in our study as we specifically included patients without prior respiratory diseases.
The reported incidence of PPC varies widely, ranging from 2.8% to 40%, primarily due to differences in the patient population and the definitions of PPC used in different studies (27-29). In our study, the incidence of PPC was observed in only three patients (2.88%). This low incidence may be attributed to the fact that the study population did not include individuals with underlying respiratory diseases.
This study has certain limitations. Regarding the lung ultrasound technique, similar to other ultrasound applications, the interpretation, identification, and quantification of B-lines and the pleural line can be subjective and subject to individual interpretation. To address this potential issue of misinterpretation, it would be beneficial to have multiple experts perform these exams and compare their findings. Additionally, due to the low number of recorded PPC, we were unable to evaluate the predictive value of preoperative ultrasonographic variables in relation to PPC.
5.1. Conclusions
Although the presence of B-lines and pleural irregularities contributes to the ultrasound interstitial syndrome, it is important to note that these findings are not specific and may be observed in patients without underlying pulmonary pathology. This factor should be taken into consideration when determining the actual incidence of abnormal pulmonary ultrasound findings in patients with a history of respiratory diseases. An ASA score ≥ 2, or the presence of systemic disease, is associated with a higher likelihood of pathological pulmonary B-lines. It is crucial to interpret ultrasound patterns in conjunction with other clinical information and consider the overall clinical context.