1. Background
Pain management after Bariatric surgery is important for the comfort of patients and surgery outcomes. After Bariatric surgery, pain is the most common complication, even if the surgery is performed laparoscopically (1). Postoperative pain control in obese patients prevents atelectasis and other lung complications, reduces patient mobility restrictions, and reduces the risk of thromboembolism. It is known that the sympathetic system plays the main role in postoperative pain (2-6). Traditionally, strong opioid analgesics such as fentanyl and their newer analogs are used for postoperative pain control. However, due to common side effects of this group of medications that can interfere with the initial healing process and lead to a delay in return to daily activities (7, 8), non-opioid analgesics such as beta-blockers and local anesthetics are used to facilitate the recovery process after surgery due to their anesthetic and analgesic effects (8-12).
Labetalol is a β-receptor antagonist suitable for intraoperative use (13). Some reports suggest this medication may have specific analgesic properties (14-17). The possibility of any intrinsic analgesic effect is yet to be elucidated, and its exact mechanism of action is unknown. Labetalol has been reported to reduce stress-related noradrenaline release in the hippocampus (18). In addition, labetalol has been shown to block tetrodotoxin-resistant sodium channels in the dorsal root ganglia (19) and modulate neurotransmission in the trigeminal nucleus of the substantia gelatinosa (20), leading to reduced afferent signaling and facilitation of the nociceptive system in the spinal cord. Finally, noradrenaline enhances heat-induced hyperalgesia in skin sensitized by capsaicin (21), suggesting that labetalol can modulate peripheral inflammatory responses. This medication can also improve hemodynamic stability during induction and recovery from anesthesia and facilitate returning to normal activities after major surgeries. The sedative and analgesic effects of labetalol can facilitate faster withdrawal from the anesthesia phase and reduction of postoperative opioid side effects such as postoperative nausea and vomiting (PONV) (22, 23).
2. Objectives
Therefore, in this study, we aimed to compare the level of hemodynamic stability during surgery and the intensity of postoperative pain between the two treatment groups of labetalol and remifentanil in morbidly obese patients who were candidates for laparoscopic surgery.
3. Methods
This was a double-blind, randomized, controlled clinical trial conducted at Hazrat Rasool Hospital in Tehran, affiliated with Iran University of Medical Sciences. This research was approved by the ethics committee of the Iran University of Medical Sciences (ethical code: IR.IUMS.FMD.REC.1400.201) and IRCT (IRCT code: IRCT20190929044924N3). The research population in this study included patients who underwent laparoscopic Bariatric surgery at this center from March 2020 to March 2022 due to morbid obesity. The inclusion criteria included:
● Absence of contraindications and sensitivity to remifentanil or labetalol.
● Age from 18 to 60 years old.
● Physical status (according to the American Society of Anesthesiologists [ASA]) of I - III for both genders).
● Definite confirmation of non-pregnancy state in female patients in the reproductive age.
● No drug addiction.
● No psychological illness.
● No history of taking beta blockers or calcium channel blockers.
Exclusion criteria included:
● Change of the surgical technique from laparoscopic to open during surgery.
● Occurrence of medication side effects, including sensitivity and anaphylaxis.
● Occurrence of unexpected responses to injectable medication, such as an excessive decrease in heart rate or blood pressure
● Occurrence of cardiac arrhythmias during surgery
After admission and one day before the surgery, patients' information was retrieved from their clinical records, and those who met the inclusion criteria and completed the consent form were included in the study. An equal number of patients were assigned to two groups of remifentanil and Labetalol through block randomization. Both patients and anesthesiologists were unaware of the injected medication for pain control and were therefore blinded.
The necessary monitoring, including electrocardiogram (ECG), heart rate, SPO2, and non-Invasive blood pressure (NIBP), was performed on the patients' entry to the operating room. Then IV line was placed, and serum therapy was started. Midazolam at a dose of 0.02 mg/kg and fentanyl at 2 mcg/kg was injected as premeditations. Then lidocaine was injected at the rate of 1.5 mg/kg. Subsequently, propofol with a dose of 2 mg/kg and cis-atracurium with a dose of 0.15 mg/kg were injected to induce anesthesia. After 4 minutes, patients were intubated with a suitable endotracheal tube and were connected to the anesthesia machine. We also used capnography for all patients after the induction and intubation. A propofol infusion pump with a dose of 100 mcg/kg/minute was installed for maintenance.
After performing the above steps, in the labetalol group, 0.15 mg/kg of the medication and 1 mcg/kg of the medication was injected before the surgical incision in the remifentanil group. Then, to maintain hemodynamic stability during the operation, these medications were repeated for patients in each group in case of an increase of more than 15% in mean arterial pressure (MAP) compared to the baseline or heart rate (HR) > 80 times per minute. The bispectral index (BIS) was used to measure the level of anesthesia. The target value in the patients was to maintain BIS in the range of 40 to 60; if it increased to more than 60, the dose of anesthetic was increased by 20 mcg/kg/minute, and if the BIS value decreased to below 40, the dose of anesthetic was decreased by 20 mcg/kg/min. The amount of anesthetic used in each patient was recorded separately. Toward the end of the surgery, only 4 mg of ondansetron was injected into all patients. If patients had a pain score of more than five after the operation, they received only one 325 mg acetaminophen suppository according to the instructions of the Laparoscopic Bariatric Surgery Department. Postoperative pain was measured in the recovery unit using the numerical rating scale (NRS). This scale is subjective, in which people verbally rate their pain on an eleven-point numerical scale. The scores of this scale are graded from 0 (no pain) to 10 (worst imaginable pain). This scale was recorded at the time of entering recovery and 30, 60, and 120 minutes after the surgery for each patient. Also, the duration of anesthesia, the duration of surgery, the duration of recovery, the dose of injected opioid analgesics, the volumes of injected intravenous fluids, and the dose of injected propofol were recorded for each patient. Post-operative nausea and vomiting were also evaluated in the recovery unit. The data was analyzed using the SPSS software (IBM, Armonk, NY, USA).
4. Results
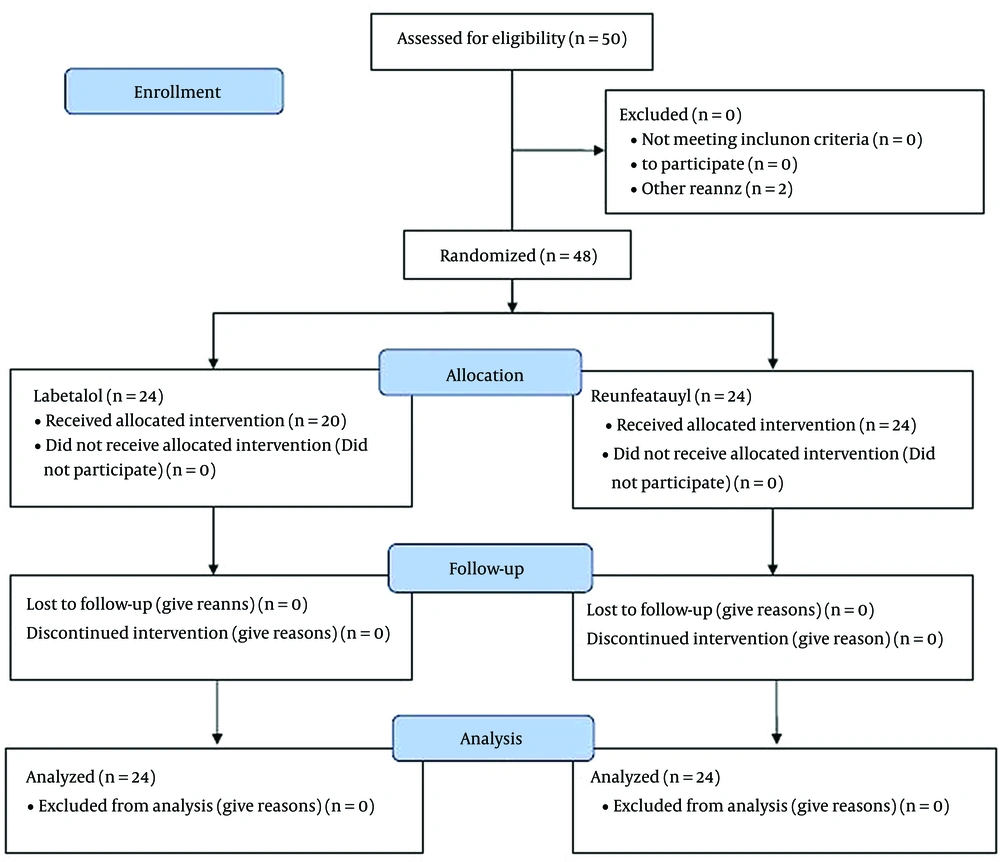
In the study, 50 patients were eligible to participate, of which 2 patients were excluded during the study period. Finally, 48 patients were examined and compared in two groups of labetalol and remifentanil (24 patients each) (Figure 1). The comparison of the basic characteristics of the patients showed that there were no significant differences between the two study groups in terms of mean age, gender, body mass index (BMI), or physical status based on the ASA index, which indicates the success of the randomization process of the patients. The mean (standard deviation [SD]) of injected midazolam was 2.31 mg (0.43) in the labetalol group and 2.20 (0.35) mg in the remifentanil group (P = 0.084). The mean (SD) amount of injected fentanyl was 247.9 mcg (40.3) in the labetalol group, and it was 237.5 mcg (37.6) in the remifentanil group (P = 0.694). The mean (SD) dose of injected propofol to induce anesthesia was 222.01 mg (25.8) in the labetalol group and 213.3 mg (23.7) in the fentanyl group (P = 0.735). Also, the mean (SD) dose of injected propofol to maintain anesthesia was 1030.8 mg (213.3) in the labetalol group and 1104.5 mg (171.6) in the remifentanil group (P = 0.131). (Table 1).
| Characteristics | Groups | P-Value | |
|---|---|---|---|
| Labetalol | Remifentanil | ||
| Age, y | 44.66 ± 8.07 | 45.37 ± 10.48 | 0.908 |
| Gender (female), % | 58.3 | 54.2 | 0.771 |
| BMI, kg/m2 | 45.62 ± 3.34 | 44.91 ± 3.75 | 0.759 |
| Physical status (ASA), % | 0.750 | ||
| I | 12.5 | 16.7 | |
| II | 62.5 | 66.6 | |
| III | 25 | 16.7 | |
| Midazolam, mg | 2.31 ± 0.43 | 2.20 ± 0.35 | 0.084 |
| Fentanyl, µg | 247.9 ± 40.3 | 237.5 ± 37.6 | 0.694 |
| Induction propofol, mg | 222.0 ± 25.8 | 213.3 ± 23.7 | 0.735 |
| Maintenance propofol, mg | 1030.8 ± 213.3 | 1104.5 ± 171.6 | 0.131 |
Comparison of Basic Characteristics of Patients and the Dose of Anesthetics Between the Two Study Groups
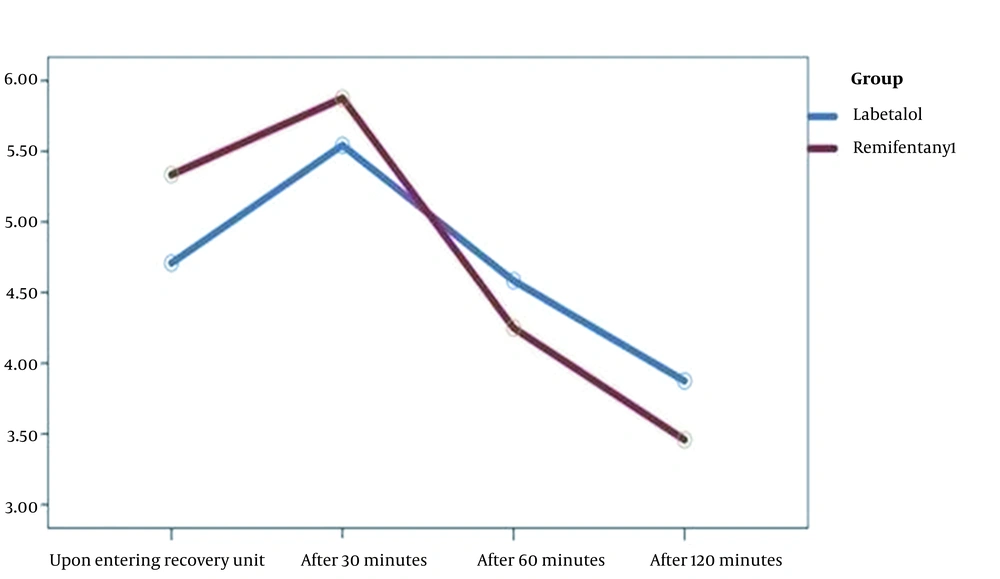
The mean (SD) duration of the surgery was 132.2 minutes (13.9) in the labetalol group and 126.1 minutes (16.3) in the remifentanil group, and this difference was not significant (P = 0.313). The mean (SD) duration of anesthesia was 151.1 minutes (15.8) in the labetalol group and 145.3 minutes (17.1) in the remifentanil group, and this difference was not significant (P = 0.462). The mean (SD) pain intensity at the time of recovery was 4.7 (2.21) in the Labetalol group and 5.33 (1.73) in the remifentanil group, and this difference was not significant between the two groups (P = 0.282). There were also no significant differences between the two groups regarding pain intensity 30, 60, and 120 minutes after the surgery (P > 0.05). In the analysis of repeated measurements, it was also found that the trend of changes in pain intensity over time did not have a statistically significant difference between the two groups. (P = 0.112) (Figure 2).
There were no significant differences between the two study groups regarding recovery time and hospital stay. The number of patients who were prescribed acetaminophen suppositories due to pain during recovery was 4 (16.7%) in the Labetalol group and 6 (25%) in the remifentanil group, and this difference was not statistically significant (P = 0.477). The frequency of PONV was 16.7% in the Labetalol group and 29.2% in the remifentanil group, and no significant difference was observed between the two groups (P = 0.303). The Rescue drug during the operation was needed in 9 people (37.5%) in the labetalol group and 16 people (66.7%) in the remifentanil group, which was statistically significant (P = 0.043) (Table 2).
| Characteristics | Groups | P-Value | |
|---|---|---|---|
| Labetalol | Remifentanil | ||
| Hospitalization length, day | 2.50 ± 1.21 | 2.37 ± 1.17 | 0.719 |
| operation time, min | 132.2 ± 13.9 | 126.1 ± 16.3 | 0.313 |
| Anesthesia time, min | 151.1 ± 15.8 | 145.3 ± 17.1 | 0.462 |
| Recovery time, min | 76.66 ± 13.18 | 83.74 ± 15.29 | 0.096 |
| Pain intensity score | |||
| Upon entering the recovery unit | 4.7 ± 2.21 | 5.33 ± 1.73 | 0.282 |
| 30 minutes later | 5.54 ± 1.10 | 5.87 ± 1.26 | 0.335 |
| 60 minutes later | 4.58 ± 1.21 | 4.25 ± 1.15 | 0.559 |
| 120 minutes later | 3.87 ± 1.17 | 3.45 ± 1.13 | 0.207 |
| Need for suppository acetaminophen, % | 16.7 | 25 | 0.477 |
| PONV, % | 16.7 | 29.2 | 0.303 |
| Need for rescue drug, % | 37.5 | 66.7 | 0.043 |
Comparison of Surgical Outcomes Between the Two Study Groups
5. Discussion
Esmolol and labetalol effectively alleviate an acute hemodynamic response to laryngoscopy and intubation (24-26). Various studies have shown that the intraoperative administration of labetalol and esmolol can obviate the need for opioids during and after an operation, the need for desflurane anesthesia, the intensity of pain in the early postoperative period, the time to the first request for pain medications, and, at the same time, can provide better hemodynamic stability. They can also improve surgical visibility, reduce blood loss during and after surgery, and even reduce surgery duration (27, 28). Our findings also demonstrated that the two groups who received remifentanil and labetalol had no statistically significant differences in pain intensity at the examined time and the pain intensity trend after the operation. This finding was consistent with previous studies (11, 29, 30). Also, regarding the frequency of the need for acetaminophen suppositories after the operation for pain control, there was no significant difference between the two groups, which further demonstrates the appropriate analgesic effects of labetalol.
In patients who undergo laparoscopic abdominal surgery, it has been demonstrated that intraoperative administration of esmolol can reduce the need for anesthetics and opioids during surgery and also the occurrence of PONV (27, 31, 32), which leads to earlier discharge from the hospital and improved patient satisfaction. However, not all authors have reported these benefits for intraoperative beta-blocker administration (33, 34). In our study, although the frequency of PONV in the labetalol group was lower than in the remifentanil group, this difference was not statistically significant. However, in a relatively similar study conducted by Lazo et al. (29), the incidence of PONV was significantly lower in patients who received esmolol than in the fentanyl and labetalol group.
While several published studies focus on the analgesic effects of esmolol in patients undergoing laparoscopic abdominal surgery, few studies describe the use of labetalol in this clinical setting. In the present study, intraoperative administration of Labetalol was an effective alternative to remifentanil to maintain hemodynamic stability during laparoscopic surgery. About the intraoperative use of opioid analgesics, this study showed that even though there was no statistically significant difference between the two groups in terms of prescribed anesthetics and analgesics to induce as well as maintain anesthesia, there were 9 patients in the labetalol group and 16 patients in the remifentanil group who needed rescue drug, which was significantly lower in the labetalol group. It showed that the patients in the labetalol group achieved better hemodynamic stability than the remifentanil group during the operation. In some studies, beta-blockers reduced the need for opioids by reducing the hepatic metabolism of medications such as fentanyl that depend on hepatic blood flow (2). These findings also support the findings of Coloma et al. (35), who reported that esmolol is an effective alternative to remifentanil for maintaining hemodynamic stability in patients undergoing elective laparoscopic gynecological surgery.
Several studies have investigated the use of beta-blockers in laparoscopic surgeries. For instance, Lee et al. (5) compared esmolol (0.5 mg/kg bolus followed by continuous infusion of 10 µg/kg/min) with ketamine (bolus 0.3 mg/kg, followed by a continuous infusion dose of 3 µg/kg/min) in laparoscopic cholecystectomy. Their results showed that esmolol more effectively reduced the need for opioids and pain scores in the early postoperative period compared with remifentanil-based anesthesia. In another study by Lopez-Alvarez et al. (36), a bolus intravenous dose of intravenous esmolol 0.5 mg/kg in anesthesia induction followed by an infusion of 5 - 15 µg per kilogram per minute was administered to patients undergoing laparoscopic cholecystectomy. The results showed that esmolol infusion reduced the need for morphine and had a more effective postoperative analgesic effect than remifentanil and ketamine. Dogan et al. (37) compared the effect of lidocaine infusion (1.5 mg/kg/min) and esmolol (1 µg/kg/min) on intraoperative hemodynamic changes, intraoperative and postoperative analgesic need and the recovery outcomes in adult patients who underwent laparoscopic cholecystectomy. They concluded that lidocaine was superior to esmolol in suppressing hemodynamic responses to tracheal intubation. In contrast, esmolol was more beneficial than lidocaine in accelerating recovery and reducing early postoperative pain. In a randomized prospective study, Collard et al. (4) compared 5 - 15 µg/kg/min esmolol infusion with intermittent fentanyl bolus doses with continuous infusion of 0.5 - 0.1 µg/kg/min remifentanil in terms of opioids needed, side effects, and postoperative hospitalization length in patients undergoing elective laparoscopic cholecystectomy. The results indicated that esmolol significantly reduced the need for postoperative opioid administration (fentanyl) and ondansetron and, as a result, facilitated earlier discharge. However, in our study, the average recovery time and hospital stay did not differ significantly between the two groups. Moon et al. (31) conducted a randomized, double-blind, placebo-controlled study to evaluate esmolol's anesthetic and analgesic effect (0.5 mg/kg bolus followed by 30 µg/kg min. infusion) in gynecological laparoscopic surgery. They observed that esmolol reduced the need for intraoperative sevoflurane anesthesia and postoperative fentanyl for pain management in the early postoperative period.
Our study also had some limitations. We used only the NRS index as the pain index in two groups, and long-term outcomes such as quality of life and long-term complications were not evaluated. Also, a potential limitation of this study was the lack of a placebo group to compare with the two active treatments for acute autonomic responses during the perioperative period. Although there was a protocol to titrate additional opioid doses in our study, the comparative doses chosen for the two study groups may have influenced the results. More importantly, the small sample size limited our ability to accurately assess outcome differences, precluding a more in-depth cost-benefit analysis.
5.1. Conclusions
The overall results showed that labetalol and remifentanil had acceptable and comparable effects on perioperative and postoperative pain with relatively low and comparable complications. However, the rescue drug needed to maintain hemodynamic stability during laparoscopic surgery was significantly lower with labetalol compared to remifentanil. These findings were related to bariatric laparoscopic surgery and may not be generalizable to other types of surgery. It is also necessary to confirm the findings of this study in future multicenter studies with larger sample sizes.