1. Introduction
Although heart surgery is preferably performed in pregnant women after delivery, it is sometimes impossible to delay the operation. The risk of maternal mortality due to extracorporeal circulation is similar to that of the general population; however, fetal mortality is estimated to be between 15% and 50% (1). Fetal complications are associated with the urgency of the surgical procedure, high-risk surgeries, fetal comorbidities, and early gestational age (2). Generally, the published cases of cardiac surgeries in pregnant patients have been reduced. Hitherto, there have been no published cases of pregnant patients with a twin pregnancy undergoing cardiac surgery or pregnant women who develop vasoplegic syndrome (VS) during cardiopulmonary bypass (CPB).
Vasoplegic syndrome is a shock characterized by normal or increased cardiac output, low systemic vascular resistance, and poor response to intravascular volume expansion and vasoconstrictor drugs. Preoperative use of intravenous heparin, angiotensin-converting enzyme inhibitors, and calcium channel blockers increases the incidence of VS. In cardiac surgery, exposure of blood to foreign surfaces in the extracorporeal circuit causes the release of inflammatory mediators such as interleukin-1, interleukin-6, and tumor necrosis factor-alpha that increase nitric oxide (NO) production and lead to an inflammatory state. Consequently, norepinephrine, epinephrine, cortisol, and arginine vasopressin are released; but these hormones are depleted in persistent shock. Thus, VS treatment includes the administration of catecholamines, vasopressin, and moderators of NO and/or anti-inflammatory medications, such as methylene blue and corticosteroids.
This clinical case report presents the results of a twin pregnant patient in whom vasoactive support and the best possible optimization of CPB led to favorable postoperative outcomes for both the patient and the fetus.
2. Case Presentation
A 33-year-old woman’s body surface area was 1.88 m2 at 15 weeks gestation, and she had a dichorionic-diamniotic twin pregnancy. Fetal ultrasonography performed at 13 weeks of gestation was normal. The patient was not taking beta-blockers, angiotensin-converting enzyme inhibitors, or calcium channel blockers. Two years before her pregnancy, she presented with infective endocarditis of the mitral valve, which required valve replacement with a bileaflet mechanical prosthesis due to perforation of the posterior mitral leaflet and tricuspid annuloplasty. Later, on the current occasion, the patient was admitted to the hospital with severe dyspnea due to stenosis of the prosthetic mitral valve (area by pressure half-time of 1 cm2, mean gradient of 17 mmHg). The anterior hemidisc was blocked, showing hyperechogenic masses adjacent to the disc and prosthetic ring. The left atrium was moderately dilated (area, 30 cm2), left ventricular systolic function (LVSF) was 60%, the right ventricle maintained a slightly depressed global systolic function (S' 6.5 cm/s, TAPSE 14 mm), and the ratio RV/LV end-diastolic area was 0.8. Determining the systolic pulmonary arterial pressure was impossible because tricuspid regurgitation was not visualized. Arterial blood gases report was: pH 7.19, bicarbonate (HCO3) 19 mmol/L, base excess (BE) -7.1 mmol/L, partial pressure of carbon dioxide (PaCO2) 53 mmHg, partial pressure of oxygen (PaO2) 80 mmHg, hemoglobin 12 g/dL. After this finding of obstruction to ventricular filling, urgent cardiac surgery was indicated. The urgency of the case limited the preoperative discussion of the plan by a multidisciplinary team, and the fetal heart rate (FHR) was not monitored throughout the operation. Still, it could be monitored at the final stages. The anesthetic and surgical preoperative plan considered the performance of those measures that could guarantee both maternal and fetal well-being.
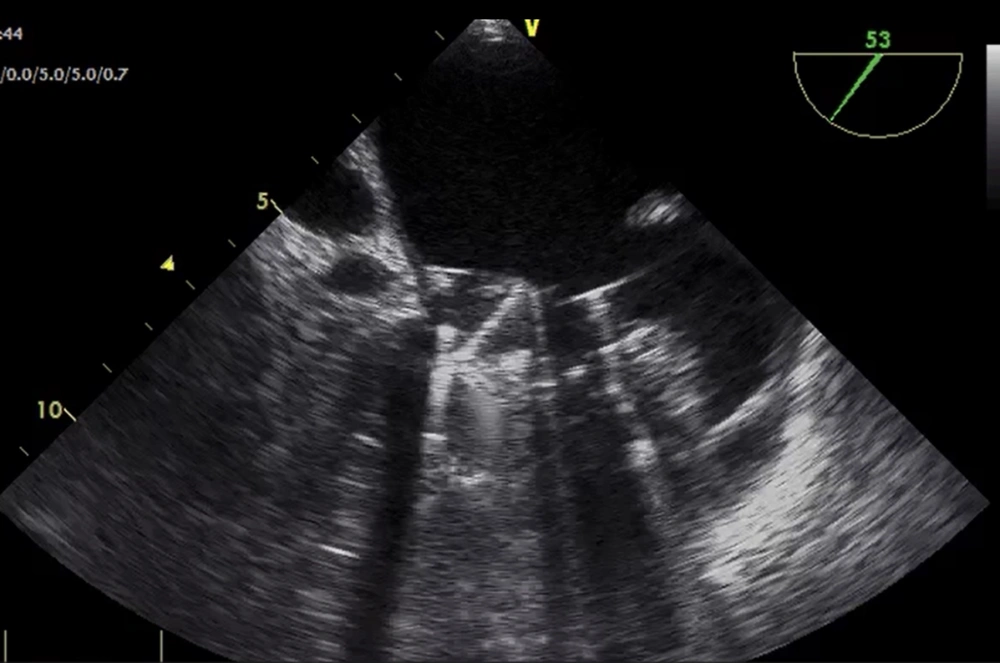
On arrival in the operating room, the patient presented with a blood pressure of 95/65 mmHg, peripheral oxygen saturation of 91%, and a sinus rhythm of 100 bpm. The patient was placed supine with a right lateral wedge to prevent compression of the inferior vena cava by the pregnant uterus. After preoxygenation and invasive blood pressure monitoring, anesthetic induction was performed with midazolam 3 mg, propofol 100 mg, fentanyl 0.15 mg, and atracurium 70 mg. Volume-controlled ventilation was initiated with a fraction of inspired oxygen (FiO2) of 60%, and a central jugular line was placed. Transesophageal echocardiography obtained a cardiac index (CI) of 2.2 L/min/m2 before the beginning of CPB, and it confirmed the findings obtained from preoperative transthoracic echocardiography (obstruction of the anterior hemidisc of the mitral valve) (Figure 1). Systemic vascular resistance (SVR) was calculated as [mean arterial pressure (MAP) – right atrial pressure / cardiac output] × 79.9 were 1278 dyn. seg.cm-5, without vasoactive support.
Before the beginning of CPB, anesthesia was maintained with sevoflurane (MAC value varied from 0.8 to 1.2%), maintaining the bispectral index in the range of 40 – 60, fentanyl 0.45 mg, and atracurium 0.4 mg/kg/h. Near-infrared spectroscopy (NIRS) cerebral oximetry monitored a regional cerebral oxygen saturation (rSO2) within 20 percent of baseline. The overall pooled mean baseline rSO2 was 60.4 ± 6.8 percent. MAP was maintained ≥ 70 mmHg. Arterial blood gases showed a mild metabolic acidosis that was corrected with bicarbonate (pH 7.3, HCO3 19.7 mmol/L, BE -6.7 mmol/L, PaCO2 39 mmHg, PaO2 242 mmHg, hemoglobin 12 g/dL). Intravenous sodium heparin (23700 Ul) was administered five minutes before arterial and venous cannulation, and activated clotting time (ACT) was maintained above 480 seconds during the procedure (measured every 30 minutes).
Cardiopulmonary bypass was initiated after cannulation of the aorta and cava veins, and the system was primed with the Viaflo Plasmalyte solution. Custodiol® solution was administered anterogradely for cardioplegia. Arterial blood gas with lactate and base deficit values were checked every 30 minutes. The objectives during CPB were a range for pH 7.35 to 7.45, pCO2 35 to 45 mmHg and (alpha-stat management without temperature correction), glucose levels <180 mg/dL, hemoglobin level > 7.5 g/dL, mixed venous oxygen saturation (SvO2) ≥ 75 percent, CPB target flow rate > 2.2 L/min/m2 to maintain MAP > 65 mmHg, mild hypothermia (temperature 34º since temperatures below 32º could lead to fetal arrhythmias, especially during overheating), MAP < 65 mmHg, SvO2 < 75 %, lactate levels > 4 mEq/L, or base deficit less than -5 were treated by increasing CPB flow rate and correcting arterial blood gas parameters.
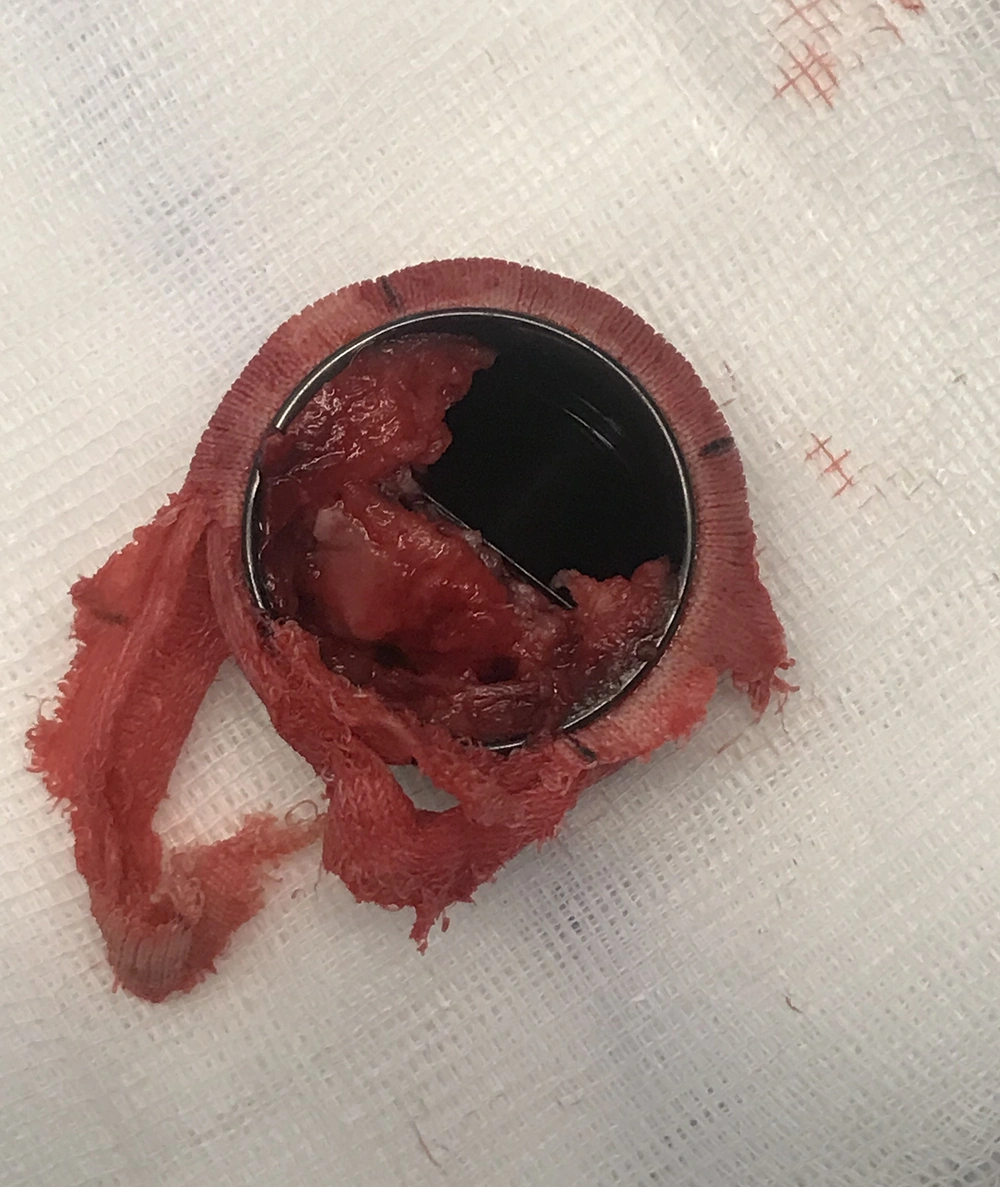
At the start of CPB, MAP dropped to 30 – 40 mmHg, SVR was 727 dyn.seg.cm-5, rSO2 decreased within 20 percent of baseline, and hemoglobin levels were 7.8 g/dL. The patient neither responded to an increase in the pump flow to 3.5 L/min/m2 nor three repeated boluses of 10 mg ephedrine. We ruled out other causes of hypotension, such as anesthetic overdose, bypass machine failure, arterial monitoring errors, aortic dissection, and unintentional torsion or clamping of the cannula. Once these causes were ruled out, norepinephrine administration was started (0.5 mcg/kg/min), achieving an elevation of MAP between 45 – 50 mmHg, and 100 mg of hydrocortisone was administered as rescue therapy. After the transfusion of two packed red blood cells, hemoglobin levels were 11 g/dL, and it was possible to reduce the norepinephrine perfusion to 0.3 mcg/kg/min, maintaining a MAP of 50 - 55 mmHg and SVR 700 - 800 dyn.seg.cm-5. Arterial blood gases showed pH 7.28, PaO2 241 mmHg, PaCO2 45 mmHg, BE -9.5, HCO3 20.6 mEq/L, and lactate 4 mmol/L. The mitral subvalvular apparatus and the obstructed prosthetic mitral valve were removed (Figure 2). The mitral prosthesis was blocked due to an overgrowth of fibrous tissue. The ischemia time was 67 min, and the total CPB time was 77 min. After aortic unclamping, the patient presented with sinus rhythm, and CPB was suspended, making it possible to completely withdraw vasoactive support for the next 15 min. Blood pressure was 140/80 mmHg, CI 3.5 L/min/m2, SVRI 1400 dyne·sec·m2/cm5, HR 80 bpm, and SpO2 99%. Intraoperative echocardiographic controls after CPB showed correct opening of the valvular discs with mean mitral valve gradients of 3.5 mmHg and no perivalvular leaks were observed. Ultrasonography and fetal cardiac activity recordings were normal at the end of the surgery. The patient was extubated 7 hours after surgery and did not present with postoperative complications. The mean mitral valve gradients increased with gestational progression to 6 – 8 mmHg, preserving systolic function without signs of pulmonary arterial hypertension. Elective cesarean delivery at term was performed, and neither the mother nor newborn presented with complications.
3. Discussion
Vasoplegic syndrome is the most severe form of hemodynamic instability. In cardiac surgery, it is associated with substantial morbidity (3), and in a patient with a twin pregnancy, it is a serious threat to maternal and fetal life. Few studies have investigated the VS during CPB, partly because there is no universally accepted syndrome definition. We used the original and most common definition of VS according to Ozal et al. (4), characterized by MAP < 50 mmHg, CI >2.5 L/min/m2, and poor response to intravascular volume expansion and vasoconstrictive drugs.
Up to 20% of patients undergoing cardiac surgery may experience VS (5), and it is unclear whether pregnancy is a risk factor. What is certain is that pregnancy entails an increase in CI, and a decrease in SVR and MAP, more marked in twin pregnancies (6). This is due to hormonal changes and high NO (7), leading to decreased vascular tone and vasodilation. These hemodynamic changes are also observed in VS and could be due to increased NO activity (8).
In our patient, the development of VS began at the onset of CPB. The CPB itself may amplify vasoplegia for two reasons. First, by releasing inflammatory mediators after the interaction of the blood with the components of the CPB, the vascular tone is deteriorated. Second, cardioplegia and hemodilution of crystalloid pump primers can reduce blood viscosity and overall vascular resistance. On the other hand, some findings seem to suggest that Custodiol® cardioplegia could be associated with the release of blood lactate immediately after aortic clamp release and with the appearance of VS in the postoperative period (9). Larger studies are required to determine the risks and benefits balance of Custodiol® in patients undergoing mitral valve surgery and in pregnant patients. However, in the current case, VS was only presented during CPB. Transient hypotension can often be observed at the start of CPB, sometimes requiring minimal vasopressor doses (10). For this reason, we initially administered a vasoconstrictor bolus. A continuous norepinephrine infusion was started immediately due to persistent hypotension and vasoplegia throughout the CPB period. Vasopressin was not administered because of the lack of confirming evidence and possible negative fetal consequences during a twin pregnancy beginning in the second trimester. This drug may lead to tonic, uterine contractions that could threaten the pregnancy. On the other hand, is well-known methylene blue has a role in attenuating vasodilation as an inhibitor of soluble guanylyl cyclase (GC) mediated by the NO pathway in the setting of inflammatory states. Still, this drug can be harmful to the fetus when administered to a pregnant woman (11). We administered corticosteroids as rescue therapy, although there was no strong evidence in this regard (12).
We were not able to maintain a MAP > 65 mmHg, such as recommended for maintaining the optimal perfusion pressure. Sun et al. (13) demonstrated that patients with MAP < 65 mmHg during CPB had a higher probability of target organ damage, such as stroke, and the risk of injury may depend on the fall in MAP and the duration of hypotension. Our results were optimal despite not reaching the MAP target (> 65 mmHg). Local vascular autoregulation phenomena probably occur, and systemic hypotension is not necessarily associated with the appearance of organic hypoperfusion. However, we cannot confirm whether this vascular autoregulation also occurs in vasoplegia, and despite potential autoregulatory mechanisms, patients who experience hypotension during CPB may have poorer outcomes (14). On the other hand, we tried not to exceed the dose of 0.5 mcg/kg/min of norepinephrine as uterine blood flow may be compromised with high doses of vasopressors (15).
Transfusion of packed red blood cells to pregnant women during CPB is controversial. The decision for transfusion should be individualized, although it seems reasonable if Hb remains < 7.5 g/dL. In our patient, the maintenance of Hb > 10 g/dL allowed us to reduce the norepinephrine dose, achieving more stable MAP values without the need for additional boluses of vasoconstrictors. The transfusion of packed red blood cells increases the viscosity of the circuit volume and could improve the hemodynamic state associated with vasoplegia.
In summary, decreased SVR and MAP during pregnancy (more marked in twin pregnancies) could contribute to a predisposition to VS during CPB. This clinical situation is clinically challenging because interventions with the greatest benefit to both the mother and the fetus that maintains a balance between the two are not well-documented. In the present case, maintaining high flows of the CPB circuit, reducing volatile anesthetic agents, administering vasoactive drugs, and optimizing hemoglobin levels above the usual thresholds improved vasoplegia and facilitated the achievement of optimal results.