1. Background
After major surgeries, patients experience pain and a long convalescence period. Restoring baseline physiologic function after surgery has always been a goal for medical staff (1). Pain is one of the most common clinical problems especially in postoperative patients, which postpones achieving this aim. Reducing pain increases patient’s satisfaction and decreases the hospitalization period (2). Moreover, relieving pain after laparotomy decreases ileus period and pulmonary complications such as mucous plaque, hypoxia, atelectasis and infection (3). Conventionally morphine is used for pain control after surgery, which has considerable side effects and exacerbates ileus and bowel dysfunction after abdominal surgeries (4, 5).
Pain receptors in injured tissues send signals, inducing a cascade which causes increased peripheral and central neuronal response. These sensory processing alterations cause an extreme response to stimulus and pain. Preventing these signals would inhibit these cascades and reduce pain postoperatively (6).
Preemptive analgesia is the use of analgesic agents before painful stimulus compared to conventional analgesia used after painful stimulus. This method was first introduced in animal studies for relieving pain after surgeries by Wall in 1988 (7) and Woolf in 1991 (8). Preemptive analgesia inhibits nocireceptors, and thus prevents the central alterations induced by afferent input after surgery. Preemptive analgesia also may reduce alterations in sensory central receptors responsible for central sensitization, neuropathic pain, hyperalgia and allodynia postoperatively (9, 10).
In cancer patients, the effect of medication on the course of disease should be considered too. Preemptive analgesia may restore the interleukin-2 level to normal earlier, which has a key role in T cells' immune response in cancer (11). Also, immunosuppressive effects of opioids used for pain control after surgery may have some tumor promoting effects in cancer patients (12); so, decreased need for opioid analgesic after surgery can provide additional benefits for use of preemptive analgesia in cancer patients.
Gabapentinoid medications are shown to be an effective analgesic in various surgeries for cancer and non-cancer patients even in eyelid surgery (13-15). Pregabalin inhibits the release of neurotransmitters such as glutamate, norepinephrine and substance P by binding to α2-δ1 protein of voltage-gated calcium channels. This change in neurotransmitters' concentration explains its various uses as anxiolytic, analgesic and anticonvulsant (16-18).
Acetaminophen is a worldwide used analgesic and antipyretic. However, there is still debate about its exact mechanism of action. It primarily inhibits COX2 and then COX1. It may also affect additional inflammatory pathways by inhibiting other peroxidase enzymes such as myeloperoxidase (19, 20).
Naproxen is an old introduced nonsteroidal anti-inflammatory and analgesic with a fully established profile of mechanism of action, adverse effects and dosing requirement. It has been shown in different studies that among nonsteroidal anti-inflammatory drugs (NSAIDs), naproxen is the one with the least effect on increasing cardiovascular risk (21-23).
2. Objectives
This study aimed to investigate the effect of preemptive oral pregabalin-acetaminophen-naproxen on pain control and morphine consumption in cancer patients after laparotomy. This combination was chosen as a noble selection for preemptive analgesia in order to block different sites in pain pathway (from production to perception).
3. Patients and Methods
This double-blind study was conducted in Cancer institute. All patients have received informed consent for being a part of this study. An informed consent form was approved by ethics in research committee of Tehran University of Medical Sciences.
Exclusion criteria included nonelective surgery, ASA physical status more than 3, history of drug sensitivity to preemptive analgesia agents, opioids addiction, non-cancerous peptic ulcers and coagulopathies (Figure 1).
A total of 40 cancer patients admitted for open abdominal surgery were recruited. We gave every patient a code according to a computer-generated list and then codes were randomized into two groups. One group received a combination of pregabalin 150 mg, acetaminophen 1 g, naproxen 250 mg (PAN) an hour before laparotomy. All patients were moved to operation hall and monitored for blood pressure, oxygen saturation, pulse rate, capnometry and ECG. All patients were premedicted by midazolam 2.5 mg and fentanyl 2 μg/kg. Anesthesia was induced by thiopental Na 3 - 5 mg/kg and atracurium 5 mg/kg. Anesthesia maintained by isoflurane 0.8% - 1.2% in 50% N2O - O2 mixture. Before skin incision 1 μg/kg fentanyl was injected. Atracurium and fentanyl were administered as needed during anesthesia. After skin closure, neostigmine (40 μg/kg) and atropine (20 μg/kg) were administered to antagonize remaining neuromuscular blockade. Then patients were moved to recovery room. After regaining consciousness completely, pain intensity was assessed using universal pain assessment tool (UPAT) (time 0). This assessment was repeated subsequently at 2, 4, 6, 8, 12, 24 and 48 hours thereafter. Morphine was administered on a protocolized schedule based on patients’ demand. Time that patient demanded first analgesic medication, total dose of morphine and other complications in the postoperative period were recorded. We also measured surgeons’ satisfaction with pain control in 24 and 48 hours after surgery with a 3-score scale ranging from completely satisfied to completely dissatisfied.
Patients and staff responsible for administering drug preoperatively, and assessing the pain level and morphine dose postoperatively, were not aware of the specific codes and intervention mode of each patient. Surgeons were blinded to the study, too.
Data were analyzed using SPSS version 16. Mean and standard deviation for all data were calculated and analyzed. We used unpaired t-test for comparing quantitative variables and chi-square or Fisher’s exact test for qualitative ones. P value less than 0.05 was considered statistically significant for all tests.
4. Results
The mean age of the patients in the PAN group was 53.45 ± 10.76 and in the control group was 56.4 ± 8.60 years, which showed no statistically significant difference (P = 0.34). Male to female ratio was 0.66 and 1 in the PAN and control groups respectively, which was not significantly different (P = 0.75). Moreover, no significant difference was found in other characteristics including body mass index (BMI), ASA status, blood pressure, time gap between diagnosis and surgery duration between the two groups (Table 1).
| Variable | PAN Group | Control Group | P Value | Statistical Significance |
|---|---|---|---|---|
| Age, y | 53.45 ± 10.76 | 56.4 ± 8.6 | 0.34 | NS |
| Gender | 0.75 | NS | ||
| Male | 8 | 10 | ||
| Female | 12 | 10 | ||
| BMI, Kg/m2 | 22.85 ± 3.33 | 22.98 ± 2.26 | 0.88 | NS |
| ASA, I/II | 10/10 | 12/8 | 0.15 | NS |
| Systolic blood pressure, mmHg | 114.5 ± 13.91 | 116.5 ± 10.89 | 0.53 | NS |
| Diastolic blood pressure, mmHg | 76.7 ± 4.9 | 79.15 ± 8.71 | 0.28 | NS |
| Cigarette smoking, No. (%) | 4 (20) | 8 (40) | 0.15 | NS |
| Non-cancerous peptic ulcer, No. (%) | 2 (10) | 0 (0) | 0.25 | NS |
| Gap between diagnosis and surgery, mon | 54.2 ± 74.48 | 55.4 ± 47.94 | 0.94 | NS |
| Surgery duration, min | 221.0 ± 83.65 | 244.0 ± 55.00 | 0.31 | NS |
Abbreviations: BMI, body mass index; NS, Not significant; PAN, pregabalin-acetaminophen-naproxen.
Fifty-five percent of all patients had gastrointestinal cancers and remaining 45% suffered from gynecologic cancers. In gastrointestinal cancer patients, affected areas were stomach, colon, pancreas and rectum/anus in 36%, 32%, 18% and 14% of the patients respectively. Also, 89% of gynecologic cancer patients suffered from ovary and adnexal malignancies and the rest 11% had uterus cancer. Most laparotomies included resection of the affected area with lymphatic resection.
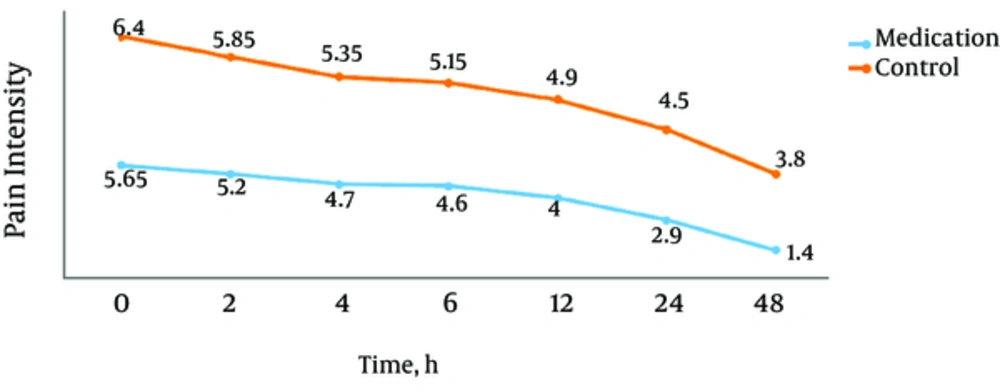
The overall mean pain intensity levels by UPAT in the PAN and control groups were 4.06 ± 0.77 and 5.3 ± 0.64, respectively which showed a statistically significant difference (P < 0.001). Pain intensity levels by UPAT at 0, 2, 4, 6, 12, 24 and 48 hours after surgery in the PAN group were 5.65 ± 1.04, 5.2 ± 0.76, 4.7 ± 0.80, 4.6 ± 0.94, 4 ± 1.02, 2.9 ± 0.96 and 1.4 ± 1.04, respectively. These values in the control group were 6.4 ± 0.59, 5.85 ± 0.93, 5.35 ± 0.67, 5.15 ± 0.75, 4.90 ± 0.79, 4.50 ± 1.10, and 3.80 ± 0.89, respectively. These scores difference was statistically significant in all time points. (P values 0.008, 0.021, 0.008, 0.047, 0.004, 0.001, and 0.001 respectively) (Figure 2).
Patients in the control group needed first opioid analgesic 70 minutes earlier than patients in the PAN group (P = 0.003).Total opioid analgesic dose administered to the PAN group was 9.9 mg (37%) less than other group, which was statistically significant (P = 0.001, 17 ± 7.6 mg and 26.9 ± 6.8 mg in the PAN and control groups, respectively). Surgeons reported more satisfaction with pain control in the medication group in 24 and 48 hours after surgery (P = 0.001) (Table 2).
| Variable | PAN Group | Control Group | P Value | Statistical Significance |
|---|---|---|---|---|
| Pain intensity mean in all points (UPAT score) | 4.06 ± 0.77 | 5.13 ± 0.64 | 0.001 | S |
| Time to demand first analgesic dose, min | 124.29 ± 88.72 | 54 ± 35.89 | 0.003 | S |
| Total morphine dose administered, mg | 17 ± 7.6 | 26.9 ± 6.8 | 0.001 | S |
| Surgeons satisfaction in 24 hours | 2.3 ± 0.66 | 1.5 ± 0.51 | 0.001 | S |
| Surgeons satisfaction in 48 hours | 2.9 ± 0.31 | 1.75 ± 0.64 | 0.001 | S |
Abbreviations: PAN, pregabalin-acetaminophen-naproxen; S, significant; UPAT, universal pain assessment tool.
In investigation of pain reduction in time periods after surgery, pain level reduction was more in time periods of 2 - 4 6 - 12, 12 - 24 and 24 - 48 hours after surgery in the PAN group compared to the control group. However, this effect was significant only in 12 - 24 and 24 - 48 hours postoperatively (Table 3).
| Time Periods After Surgery | PAN Group | Control Group | P Value | Significance |
|---|---|---|---|---|
| 0 - 2 | 0.45 ± 0.6 | 0.55 ± 0.68 | 0.62 | NS |
| 2 - 4 | 0.5 ± 0.688 | 0.05 ± 0.607 | 0.15 | NS |
| 4 - 6 | 0.1 ± 0.553 | 0.2 ± 0.69 | 0.618 | NS |
| 6 - 12 | 0.6 ± 0.68 | 0.25 ± 0.55 | 0.08 | NS |
| 12 - 24 | 1.1 ± 0.71 | 0.4 ± 0.681 | 0.003 | S |
| 24 - 48 | 1.5 ± 1.06 | 0.7 ± 0.59 | 0.001 | S |
Abbreviations: NS, not significant; PAN, pregabalin-acetaminophen-naproxen; S, significant.
We compared pain intensity at 2, 4, 6, 12, 24, 48 hours after surgery to time 0 in both groups. Pain reduction was more in 12, 24 and 48 hours periods after surgery in the PAN group compared to the control group. However, this was significant only in 24 hours and 48 hours after surgery (Table 4).
| Hours After Surgery | PAN Group | Control Group | P Value | Significance |
|---|---|---|---|---|
| 0 - 2 | 0.45 ± 0.6 | 0.55 ± 0.68 | 0.62 | NS |
| 0 - 4 | 0.9 ± 0.75 | 1.05 ± 0.51 | 0.628 | NS |
| 0 - 6 | 1.05 ± 1.14 | 1.25 ± 0.55 | 0.486 | NS |
| 0 - 12 | 1.65 ± 1.80 | 1.5 ± 0.5 | 0.58 | NS |
| 0 - 24 | 2.75 ± 0.85 | 1.90 ± 0.85 | 0.003 | S |
| 0 - 48 | 4.25 ± 1.11 | 2.60 ± 0.59 | 0.001 | S |
Abbreviations: NS, not significant; PAN, pregabalin-acetaminophen-naproxen; S, significant.
Most common complication in first postoperative 24 hours in our study was nausea, followed by somnolence and dizziness. All these three complications in the PAN group were more than the control group, but this difference was not statistically significant (Table 5).
| Complication | PAN Group | Control Group | P Value | Significance |
|---|---|---|---|---|
| Nausea | 75 | 65 | 0.37 | NS |
| Somnolence | 70 | 50 | 0.17 | NS |
| Dizziness | 60 | 40 | 0.38 | NS |
Abbreviations: NS, not significant; PAN, pregabalin-acetaminophen-naproxen.
aValues are expressed as percentage.
5. Discussion
In this clinical trial, preemptive use of PAN was associated with reduced pain intensity at 0, 2, 4, 6, 12, 24 and 48 hours after surgery significantly. The total administered morphine dose in the PAN group was 37% less than the control group.
In terms of postoperative pain and reduced need for opioid by preemptive analgesia, similar findings are reported in different surgeries in other studies. Preemptive gabapentin on patients scheduled for laparoscopic cholecystectomy, lumbar discectomy, arthroscopic knee and dacryocystorhinostomy repair yielded significant opioid sparing and decreased pain score (24-27). In two other studies conducted on tonsillectomy and total abdominal hysterectomy, pain intensity difference was not significant between the gabapentin and placebo groups; however, the need for opioids reduced in both studies (28, 29). A study on patients undergoing abdominal hysterectomy due to benign diseases, has reported 36% less pain score but no significant opioid consumption difference (30). Arguably administering preemptive gabapentinoid drugs can decrease postoperative pain and need for opioid analgesics (31).
We used pregabalin, acetaminophen and naproxen together in our study. This is the first study to investigate the use of this three-agent preemptive analgesia. Different medications are investigated for preemptive analgesia. Using combination of various agents and blocking more than one pathway has yielded more promising results. Combination of gabapentin and refecoxib is shown to be superior to use of single agent in total abdominal hysterectomy patients (32). Concurrent use of acetaminophen and naproxen is shown to increase analgesic efficacy in a systematic review analyzing 21 human studies (33). It’s suggested that pregabalin with naproxen can have a synergic or at least additive anti hyperalgesia effect by experimental study on rats and nocireceptors thresholds (34). Riad et al. in his investigation on children scheduled for surgery due to inguinal hernia reported that preemptive use of acetaminophen and diclofenac suppository would reduce postoperative pain and need for analgesics more than patients receiving single agent (35). Another recent systematic review has shown opioid sparing effect for preemptive use of acetaminophen and NSAID in controlling pain after surgery (36).
In most of these studies, researchers used visual analogue scale (VAS) for assessing pain intensity. However, now it is a debate whether some people might report intensity of stimulation instead of pain perception in visual analogue score. Other tools included faces scales such as Wong and Baker pain scale for children, verbal numerical rating scale, or a scale made by authors. To assess pain, we used UPAT, which is newly designed and a combination of visual analogue scale, faces scales and activity tolerance scale (37, 38).
In our study pain intensity in all time points was significantly less than the control group, and most prominent pain reduction was seen in an interval of 12 - 48 hours postoperatively. Pain level reduction in intervals earlier than 12 hours after surgery was not significantly different between the two groups. Similar results were reported by other researchers. In a study on laparoscopic cholecystectomy, a group of preemptive gabapentin had lower pain scores in time intervals of 0 - 6, 6 - 12, 12 - 18, 18 - 24 compared to the tramadol and placebo groups (27). Eman et al. reported that preemptive pregabalin in total abdominal hysterectomy reduced pain significantly in a period of 4 - 24 hours after surgery. However, no significant difference was observed in the pain score at 1 hour after surgery (16). This insignificance in early hours after surgery may be explained by limited activity of patient in these hours. Possibly walking of patients in later hours increases intra-abdominal pressure, which stimulates pain receptors and thus highlights the difference between two groups.
Time to demand first analgesic dose was significantly longer in PAN group in our study. Although in many studies, this item was not investigated, Similar studies yielded consistent results (16, 39, 40). However this effect was smaller in those studies and not statistically significant. This difference can be attributed to our multimodal preemptive analgesia, in these two other studies only preemptive pregabalin or pregabalin with a NSAID have been used in contrast to our three agent modality. Blocking of more pain pathways possibly delays the time to feel first signs of post-operative pain.
In our study, most common complications were nausea, somnolence and dizziness which did not show a significant difference between the two groups. Pregabalin complications include nausea, somnolence, dizziness, ataxia, diplopia, and weight gain, which are mostly seen in chronic use (41). Preemptive pregabalin was associated with similar side effects in other studies. Sedation, nausea/vomiting, dizziness, gait disturbance have been reported in studies on single agent gabapentinoid drug (27, 29). We used 150 mg pregabalin in our study, which is the major drug implicated in more nausea/vomiting and somnolence in the case group. Various studies have been conducted on determining the optimum dose of pregabalin for preemptive analgesia. In a clinical trial by jokela, pregabalin 150 mg with 800 mg ibuprofen was superior to pregablin 75 mg with ibuprofen 800 mg in terms of controlling pain without significantly increased side effects (40). In another clinical trial by jokela, pregabalin 600 reduced postoperative analgesic need more than pregablin 300 mg, but caused considerable significant side effects of dizziness, headache and blurred vision (39). Pandey showed that increasing gabapentin dose more than 600 mg, does not affect postoperative pain in lumbar discectomy (26). It seems that specific type of surgery should be considered in settling the recommended preemptive pregabalin dose. However, in studies on preemptive NSAIDs especially in children, bleeding is a concern, (36) in our study no perioperative bleeding is noted in the PAN group. We should note that in decision to evaluate preemptive analgesia side effects, avoiding opioid noticeable side effects such as respiratory depression should be taken in account.
Despite different investigations on preemptive analgesia, best choice of drugs, efficient dose and time to use them, possibility of multiple dosing or continuing medication after surgery are not completely determined. More investigations are needed for making guidelines about preemptive analgesia in specific surgeries and age groups.