1. Background
Urinary tract stones are common painful causes for referring to emergency departments (1, 2). Patients’ satisfaction regarding treatment quality at the emergency department (ED) largely depends on how their pain is managed (3-5).
Non-steroidal anti-inflammatory drugs (NSAIDs), such as ketorolac, are good selective treatments for pain alleviation in patients with renal colic (6). However, certain issues such as nephropathy, headache, dizziness, gastrointestinal mucosal irritation, and bleeding are among complications. Following their usage, lithotripsy may be postponed because of their effect on platelet function (7).
Desmopressin is a synthetic analog of the anti-diuretic hormone. This drug is available in the form of intranasal spray, with quick onset of action and little side effects. While it seems that the main mechanism of action of desmopressin is lowering intraurethral pressure, it is likely to cause relaxation of renal, pelvic, and ureteral muscles, and pain alleviation via direct action on these muscles. The third mechanism of action of desmopressin is its central analgesic effect through releasing beta-endorphin from hypothalamus. Currently, desmopressin is used as an adjuvant therapy and a promising alternative for patients with renal colic, especially those for whom opioids cannot be used or those, who are refractory to initial treatment of renal colic (8).
2. Objectives
This study aimed at comparing the effect of intranasal desmopressin with that of intravenous ketorolac in pain management of patients with renal colic, referring to the emergency department.
3. Methods
3.1. Study Design
This was a double-blind randomized clinical trial, conducted during 2014 to 2016 at the emergency departments of Loghman Hakim, Imam Hossein, and Shohada Tajrish Hospitals, all of them located in Tehran, Iran. The ethics committee of Shahid Beheshti University of Medical Sciences approved the protocol of this study (code IR.SBMU.SM.REC.1395.79). The patients were included voluntarily and informed consent forms were obtained. Throughout the study, researchers were committed to the principles of the declaration of Helsinki. The protocol of this study was registered in the clinical trials registry (www.clinicaltrials.com) with registration code of NCT02937896.
3.2. Participants
The patients in the age range of 16 to 50 years old with renal colic diagnosis by an emergency medicine physician were included in this study. The impression of renal colic was considered based on patient’s signs and symptoms; subsequently, it was confirmed using computed tomography or ultrasound. Patients with history of allergic reactions to desmopressin or ketorolac, hypertension, coronary artery disease, peptic ulcer disease, kidney failure, liver failure, coagulopathy, anticoagulant therapy, influenza, rhinitis, asthma, addiction, pregnancy, and lactation were not eligible. Use of analgesics within 4 hours and alpha-blockers before admission, history of surgery of the kidney or ureter, and fluids therapy immediately before admission were considered as exclusion criteria. Those, who could not bear the pain and did not want to continue, were also excluded.
Considering α = 0.05 and statistical power β = 0.9, in order to find a significant difference of at least 1 in pain alleviation between the two groups with a statistical power of 90%, the sample size for each group was determined as 20 people.
3.3. Randomization and Blinding
By using a computer-generated code, eligible patients were randomized between two groups, A and B, in a 1:1 ratio. Color and appearance of the drugs were identical. The medication was prepared by one nurse and administered by another, who was blinded to the aim of the study. Therefore, the patients, nurses, and physicians were all blinded to identity.
3.4. Primary Assessment
First, using a 10-centimeter visual analog scale (VAS), all participants were assessed for severity of pain. To assess the pain intensity based on VAS, patients marked the score that best described their pain intensity along a 10-cm linear scale, marked at one end with a term such as no pain, and at the other end with worst imaginable pain. Pain intensity was measured in millimeters from the no-pain end. A difference of 13 mm was the minimum clinically significant change reported by patients, whereas, an average decrease of 30 mm appeared to be the minimum acceptable change for pain control (9).
3.5. Intervention
In Group A, 40 μg of intranasal desmopressin spray (Minirin, Ferring, Kiel, Germany, 500 μg/vial, 10 μg/puff) equal to 4 puffs of the available product (each puff contains 10 micrograms in a nostril alternately) combined with 1 cc of stilled water was administered. This dose was considered based on prior works on the same topic (10-12). Group B received 30 mg of intravenous ketorolac (ExirPharma co, Tehran, Iran, 30 mg/cc) and NACL 0.65% (Decosalin 0.65%, Raha Company, Iran, 20 mL nasal spray) intranasal spray (4 puffs; each puff in a nostril alternately).
3.6. Outcome
All participants in both groups were assessed for severity of pain according to VAS standards 10, 30, and 60 minutes after drug administration. Decreasing of 3 or more scores was considered significant. In a situation of pain intensity equal to 5 or more after 30 minutes, 5 mg morphine sulfate was administered intravenously as rescue therapy. If any degree of pain persisted after 60 minutes, an additional dose of morphine sulfate was administered.
3.7. Data Gathering
The data gathering tool was a checklist, in which the patients’ demographic information and also information regarding the patients’ history and follow-up were recorded.
3.8. Analytical Analysis
The obtained data were statistically analyzed using SPSS v.22. Mean ± standard deviation (SD), together with frequency and percentage, were used to describe the descriptive data. The statistical tests of analysis of variance (ANOVA) and t test were applied to compare the pain scores of the two groups at different times. In order to compare the mean pain scores at different times, paired t test and Bonferroni Correction test were used.
4. Results
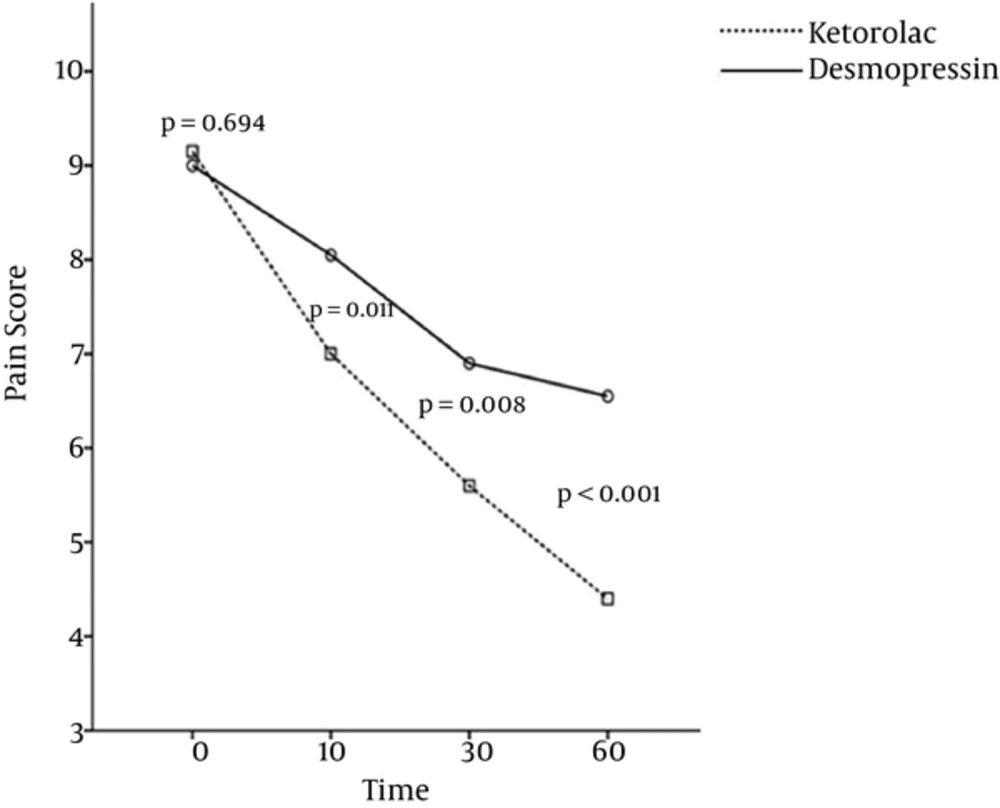
Overall, 40 patients with mean age of 32.53 ± 6.91 (21 to 49 years old) participated in this study (72.5% of them were males). The patients’ demographic and baseline findings are summarized in Table 1. Gender ratio (P = 0.288) and mean age (P = 0.165) of the patients in the two groups were not significantly different. The mean pain scores at zero time (P = 0.694) had no significant difference, yet, the mutual effect of therapy and treatment group was significant (P < 0.001), which meant that the procedure of the pain scores’ changes in two treatment groups had a significant difference, such that reduction of mean pain scores in the ketorolac group was steeper than in the desmopressin group (Figure 1). The mean pain scores after 10, 30, and 60 minutes from drug administration in the ketorolac group was significantly lower than the desmopressin group.
| Variable | Desmopressin | Ketorolac | P Value |
|---|---|---|---|
| Age | 31.0 ± 6.5 | 34.1 ± 7.1 | 0.165 |
| Gender | 0.288 | ||
| Male | 13 (65) | 16 (80) | |
| Female | 7 (35) | 4 (20) | |
| Pain score | |||
| On arrival | 9.0 ± 1.4 | 9.2 ± 1.1 | 0.694 |
| 10th min | 8.1 ± 1.3 | 7.0 ± 1.2 | 0.011 |
| 30th min | 6.9 ± 1.4 | 5.6 ± 1.5 | 0.008 |
| 60th min | 6.6 ± 1.3 | 4.4 ± 1.8 | < 0.001 |
aValues are expressed as men ± SD or No. (%).
According to the findings, the time effect was statistically significant in each group, which means that, in each treatment group, the mean pain score showed a significant reduction over time. In none of the two groups, side effects resulting from drug administration were reported.
5. Discussion
According to the findings of the present study, although intranasal desmopressin leads to significant pain control in patients with renal colic referring to the emergency department, yet, it seems that its effect is less in comparison with intravenous ketorolac.
Based on the literature, in visual analog scale, a 13-mm reduction in pain severity is considered significant, whereas a 30-mm reduction is supposed to be acceptable (13). Accordingly, and given the results of this study, one can conclude that desmopressin intranasal spray has relative effectiveness in pain management of patients with renal colic and may be used as an adjuvant non-injectable drug in pain management of these patients, which is in accordance with the results of previous studies.
In a study by Salam et al. in 2013, one half of 126 studied patients were fully relieved from pain 30 minutes after using intranasal desmopressin spray and did not need any additional analgesics. There were no reported side effects resulting from this drug in that study (14). In this study, desmopressin did not result in full pain alleviation, though it led to a significant reduction in patients’ pain. El-Sherif et al. compared the effectiveness of intranasal desmopressin spray with that of intramuscular diclofenac, and reported that 94.4 percent of patients receiving desmopressin were fully relieved from pain 30 minutes after drug administration (15). The findings of this study somewhat criticize the results obtained by El-Sherif et al.; perhaps it can be said that the results of this study are more compatible with the results obtained by Kumar et al., who confirmed the role of desmopressin in management of mild pain of renal colic within 30 minutes after administration (16). Of course, in contrast to these studies, which mostly confirmed the efficacy of desmopressin, in a study by Alibeigi et al. which was conducted to compare the effectiveness of this drug with that of pethidine, it was shown that desmopressin was not effective in pain management of patients with renal colic (17).
Given the existing controversies, some of which were noted, future studies should evaluate the effectiveness of desmopressin on final extent of kidney stone excretion, different forms of desmopressin in pain management, and satisfaction of patients with renal colic.
5.1. Conclusions
It is likely that desmopressin is less efficacious than ketorolac, though desmopressin leads to a significant alleviation of pain in patients with renal colic.