1. Introduction
Charcot-Marie-Tooth disease (CMTD), the most common form of inherited peripheral neuropathy in children, is a slowly progressing disease presenting with persistent weakness, distal muscle wasting, sensory loss, early reduced or lost deep tendon reflexes, and neuropathic pain. The most common associated symptoms are physical disability, muscle spasm, and pes cavus, which impact the patient’s quality of life. Therefore, these patients usually need multiple surgeries for correction of soft tissue and bony structures deformities.
Anesthetic considerations in CMTD include adverse reactions to neuromuscular blocking drugs (NMBD), rare susceptibility to hyperthermia, and a protracted response to some intravenous anesthetics. Moreover, these patients are at risk for respiratory insufficiency after general anesthesia (GA) due to phrenic nerve involvement, obstructive sleep apnea, or prolonged muscle weakness (1). Mechanical ventilation dependency for up to a month after GA has been reported in CMTD (2). Patients with kyphoscoliosis suffer from restrictive lung disease and decreased exercise tolerance. The severe cases also represent impaired arterial blood gas analysis due to their chest deformity. Chronic hypoxemia can result in corpulmonale, pulmonary hypertension, and right ventricular hypertrophy.
The severity of these abnormalities depends on the cobb’s angle. Cardio pulmonary function decreases significantly in cases with a scoliosis angle of more than 100°. In addition, at a more cephalad location and more involved vertebrae, the cardio pulmonary abnormality will be more severe. Laryngoscopy and intubation might be problematic in severe cases, due to lateral displacement and rotation of trachea. The likelihood of post anesthesia respiratory insufficiency needs special anticipation and precaution in CMT. This risk may be more serious when the patient is not intubated in the post-operative period and needs narcotic analgesics.
Neuraxial anesthesia in CMT adult patients has been reported in the literature earlier, however, we could not find any paper in favor or against neuraxial anesthesia in CMT pediatrics. Combination of epidural and light general anesthesia in the reported patient with major risk of respiratory insufficiency could safely decline the risk of post-operative respiratory complications.
2. Case Presentation
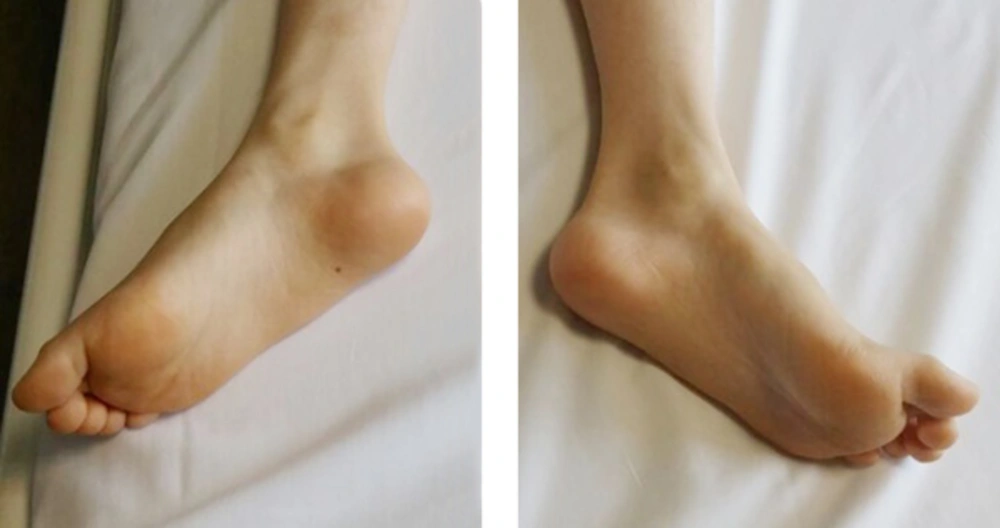
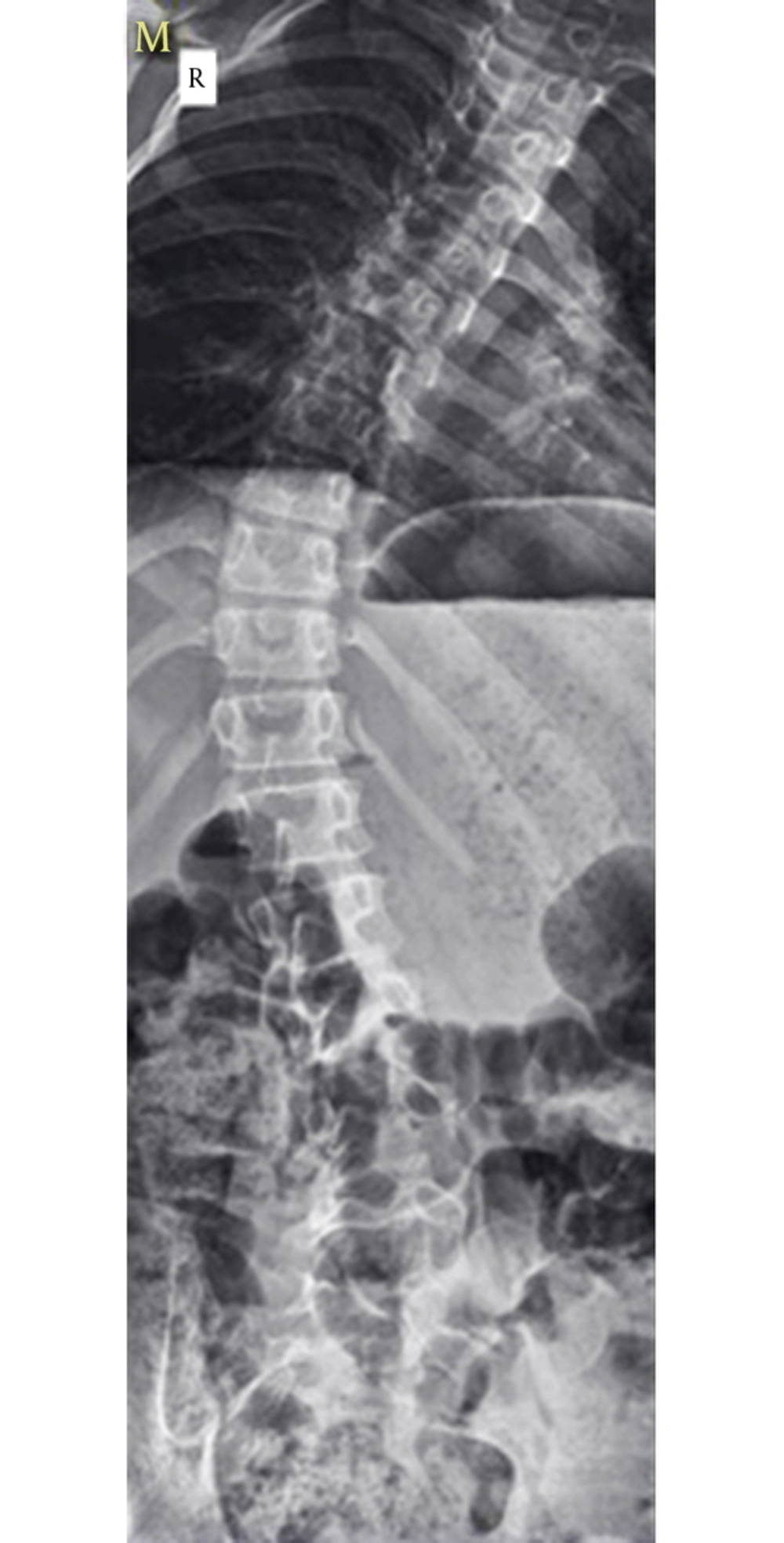
A 12-year-old male with CMTD (weight 23 kg and height 130 cm) was scheduled for thoracolumbar kyphoscoliosis surgical correction. He had a history of childhood asthma, which resolved at about 3 years of age. He also had beta-thalassemia minor and hypoplastic kidneys. He had a history of GA for umbilical hernia repair surgery 5 years ago, where he experienced a delayed awakening from anesthesia and respiratory function impairment, in which his ventilation was supported mechanically for 5 hours in the post anesthesia unit while he was intubated due to insufficient respiratory force. On his preoperative evaluation, chest and lower limbs deformities were obvious (Figures 1 - 2). Spirometry showed a moderate restrictive pattern with FVC about 65% of the predicted value. All laboratory tests were within normal range. In the physical examination, neck extension was reduced, however, airway assessment was near normal otherwise. Heart sounds were normal and the lungs were clear and equal bilaterally. Continuous EA as the main part of intraoperative analgesia combined with GA to secure airway and maintain ventilation was planned for this surgery.
After getting informed consent, standard monitoring and oxygenation with Hudson mask began in the operating room followed by intravenous light sedation provided with fentanyl and propofol. Epidural catheter (B. Braun Perifix ONE epidural set 18G) was introduced into his L4-L5 intervertebral space in the lateral decubitus position without difficulty after skin preparation and draping. We chose this intervertebral level for catheter insertion due to the fact that it was away from the surgical field. Then, the catheter was threaded about 15 centimetres beyond the needle tip guided by ultrasound following a test dose of 0.1 mL/kg lidocaine 1.5%. No paraesthesia was elicited during this stage. A compound ultrasound system (Medison Samsung, SonoAace X8) and a 5 MHz curved array transducer were used for guidance. The ultrasound revealed the catheter tip at about the lower margin of the 9th thoracic vertebra. A total volume of 18 mL lidocaine 1% with 1:200.000 epinephrine was injected incrementally into the epidural catheter. Almost 15 minutes later, analgesia was confirmed within the surgical field by the pinprick test.
Then, following administration of premedication in supine position, anesthesia was induced by propofol (2.5 mg/kg) and atracurium (0.45 mg/kg). After inserting and fixation of a cuffed endotracheal tube sized 6 and placement, pressure control mode ventilation was started and light anesthesia was maintained with isoflurane 0.8% and oxygen 75%. An arterial cannula was fixed in his radial artery for invasive blood pressure monitoring.
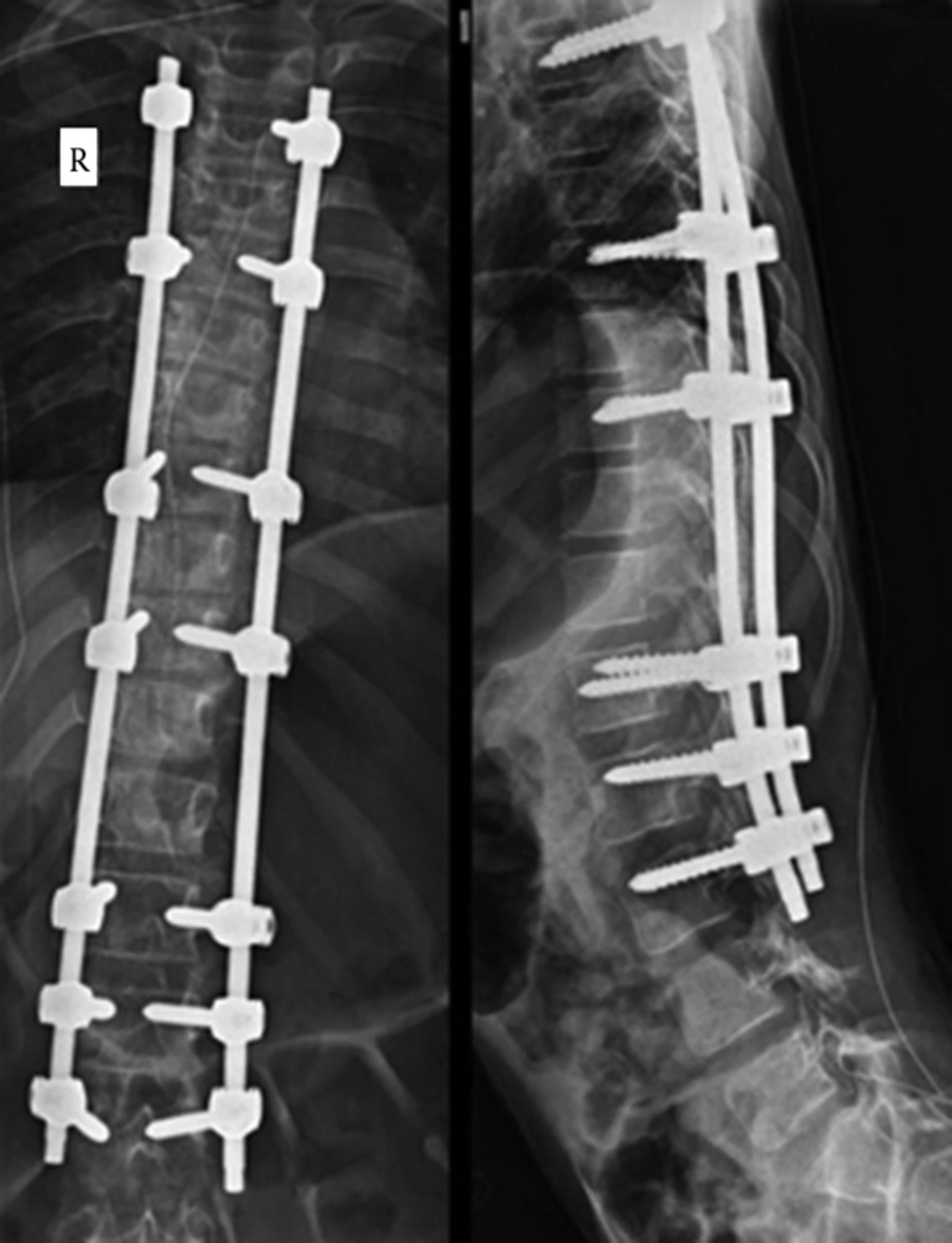
Longitudinal incision was made from T2 to L4 by the surgeon following changing the position into prone. Long thoracolumbar deformity was corrected by posterior instrumentation and fusion from T3-L3 by pedicular screws and titanium 5.5 rods (Figure 3). Intraoperative blood loss was within the expected range and the operation proceeded uneventfully. Neuromuscular blockers were not repeated during the surgery. Finally the paralysis was reversed by neostigmine (1.5 mg) and atropine (0.75 mg). The whole operation lasted for 4.15 hours, during which the epidural catheter was charged 3 times with 6 mL bupivacaine 0.25% for each time. The patient was extubated and observed in the recovery area for 30 minutes, during which he fully regained his consciousness. His verbal analog pain score (VAS) was 3 out of 10 and Ramsay sedation score (RASS) was 2 out of 6 before being transferred to the general ward. The patient received 0.125% bupivacaine (4 mL/hour) as “patient controlled epidural analgesia” for the next 2 days and was discharged on the 4th postoperative day. One month later, in his follow-up session, he had no new neurological problems.
3. Discussion
There is still shortage of information regarding choosing the best anesthesia for patients with CMTD. The main concerns of anesthesiologists are unpredictable response to hypnotics or muscle relaxants, cardiac dysrhythmias, postoperative respiratory failure, and exacerbation of neurological symptoms. Some patients with CMT are more sensitive to thiopental. Thus, the dose of thiopental should be reduced and the amount of reduction is strongly related to the severity of nervous system impairment (3). However, volatile anesthetics and propofol are recommended and can be administered safely. While there are concerns regarding succinylcholine induced hyperkalemia, Antognini reported some cases without this adverse effect (4). Prolonged neuromuscular blockade by NDMR has been reported (5). However, normal responses have also been observed (6).
While published reports support the safety of peripheral and neuraxial methods of anesthesia in CMTD without aggravating the disease (7, 8), there are still concerns regarding exacerbation of pre-existing neurological deficits or new neurologic complications following RA in CMTD. Reduced intraoperative opiate requirements and diminished postoperative respiratory depression are benefits of applying regional anesthesia in CMTD (6).
In our literature review, immediate or long-term neurological complication related to neuraxial anesthesia has not been reported in CMT. Single injection or continuous spinal anesthesia have been applied safely for adult CMT patients (9, 10). There are also few reports describing epidural anesthesia for labor or orthopedic surgeries in adult CMT patients without neurological adverse effect (11, 12). However, epidural anesthesia has not been reported in CMT pediatric patients so far.
Generally, combination of RA with GA in a variety of patients provides more safety, more rapid recovery from anesthesia, better post-operative pain management, and less complication related to muscle relaxants compared to sole GA (8, 13-15). Combined epidural/general anesthesia (CEGA) has been earlier recommended for spine surgery. This combination affords better hemodynamic condition, less blood loss, and optimal surgical condition and post-operative pain management (15), however, we did not find any data recommending the use of combined epidural/general anesthesia in pediatrics with CMT. For this boy, which went under the surgery in the prone position, we combined light general anesthesia to secure airway and maintain ventilation, with intense epidural anesthesia to provide analgesia, spinal muscles relaxation, and controlled hypotension.
Doubts against neuraxial anesthesia in CMT come from the experience with multiple sclerosis (MS), while MS is a central neuropathy and CMT is only peripheral. Due to a lack of data addressing the cost and benefit of regional anesthesia in CMT patients, recommendation of RA in patients with CMT is uncertain (7, 8). Despite lack of convincing evidences, RA may be blamed for any post-operative neurological deterioration in cases with CMT. Therefore the anesthesia plan should be chosen in combination with the patient following precisely defining the natural course of the disease.
3.1. Conclusion
Severe scoliosis, restrictive lung disease, and the history of prolonged respiratory insufficiency after short duration anesthesia were some of the major risks associated with GA in this patient. CEGA in this patient was safe and also reduced life threatening risks. The benefits of regional anesthesia in our patient were considered to outweigh the theoretical risk of further neurological damage. CEGA avoided some of the problems associated with CMT disease such as delayed awakening and post-operative respiratory insufficiency. Data from large trials supporting the safety of neuraxial in CMT does not exist; therefore, we propose larger studies in this group of patients into its safety and benefits.