1. Background
Total intravenous anesthesia (TIVA) has been accepted as a viable alternative to the traditional balanced anesthesia with volatile anesthetics (1, 2). TIVA provides safe and fast induction, maintenance and termination of general anesthesia, followed by short recovery times, less postoperative nausea and vomiting (PONV) and fast dismission from the hospital. TIVA is most frequently done by combining propofol with remifentanil, because of the fast pharmacokinetics due to its short half-life. The drawback of this is the lack of residual analgesic effect after termination of the continuous infusion. Additionally, remifentanil is more expensive than other short acting opioids such as alfentanil or sufentanil.
2. Objectives
To optimize the management of our patients for minor procedures such as breast surgeries, we developed a protocol for TIVA with propofol and either alfentanil or sufentanil. The primary aim of the current study was to compare postoperative analgesia after total intravenous anesthesia with either alfentanil or sufentanil. Secondary aims were the hemodynamic stability of the patients during the case and the time needed for anesthesia emergence.
3. Patients and Methods
After approval by the local ethics committee, written informed consent was obtained from 38 female ASA I to III patients scheduled for elective breast surgery (excisions, ablations, axillary dissections). Exclusion criteria were defined as: history of severe chronic neurological, cardiac or pulmonary diseases, history of drug abuse, chronic preoperative use of analgesics, contraindications against any of the drugs used in the study, contraindications against laryngeal mask airway, and pregnancy.
As per hospital standard, all patients received oral premedication with 20 mg temazepam the evening before and 7.5 mg midazolam approximately 60 minutes before induction of anesthesia. Randomization was performed on the day of surgery by means of a predefined randomization list which was only known to the principal investigator (PI; author PS). In order to ensure allocation concealment, the PI did not recruit patients and did not participate in the anesthetic.
After randomization, an anesthesiologist who was not involved in the planned procedure prepared the blinded study medication by diluting either 5,000 µg alfentanil or 50 µg sufentanil with saline in a 50 ml syringe, which resulted in equipotent dosing of either alfentanil 100 µg ml-1 or sufentanil 1 µg ml-1 (assuming that the equianalgesic dose ratio is 100:1 for alfentanil versus sufentanil) (3, 4). The anesthesiologist who did the TIVA was blinded to the content of the syringe; dosing was done by volume (e.g. mlkg-1 and mlkg-1 hr-1) to maintain blinding.
On arrival in the operating room, standard monitoring of vital parameters was established (ECG, oxymetry, non-invasive blood pressure; Dash 3000 monitor, Marquette). A bispectral index measurement for estimation of hypnosis (BIS; BIS-monitor, Aspect Medical) was started, and baseline values of all these parameters were recorded. A venous catheter was inserted and 8 10 mlkg-1 of crystalloid fluid was given. Glycopyrollate (0.2 mg) was administered to reduce salivation.
After preoxygenation, a loading dose of propofol (2.5 mg kg-1) was administered followed by a loading dose of the study drug (20 µg kg-1 alfentanil or 0.2 µg kg-1 sufentanil). After three minutes, a laryngeal mask was inserted and mechanical ventilation was established (volume-control, tidal volume 6 mlkg-1, PEEP of 5, rate 10-16 min-1 adjusted to achieve an end-tidal CO2 of 35 mmHg). Each patient received a rectal dose of 100 mg diclofenac after induction of anesthesia.
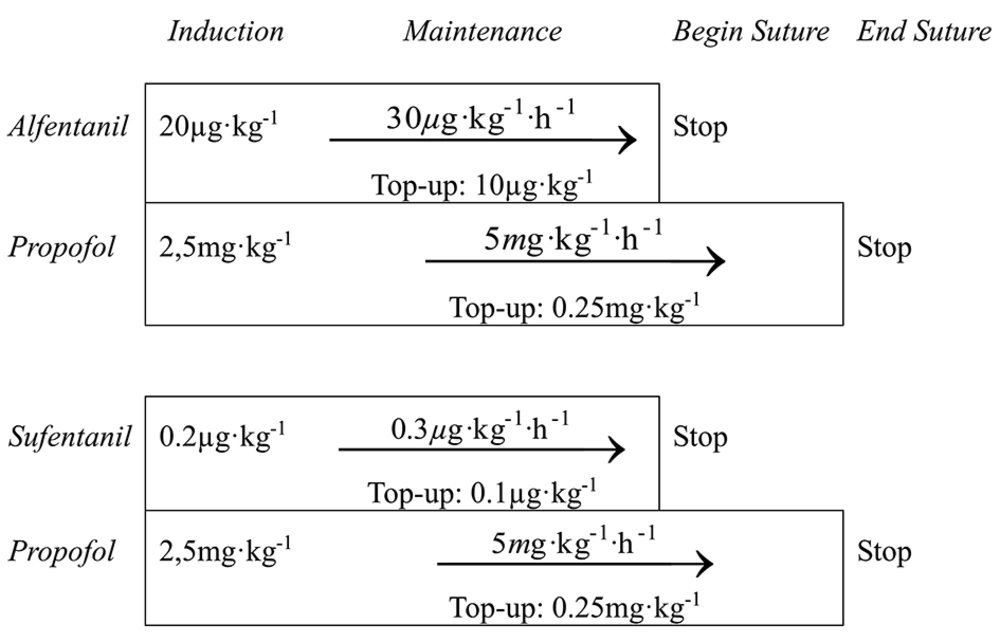
Anesthesia was maintained with 5 mg kg-1 h-1 propofol and 30 µg kg-1 h-1 alfentanil or 0.3 µg kg-1 h-1 sufentanil, respectively (Figure 1). Crystalloid fluid replacement was administered at 5-10 mlkg-1 h-1. BIS was used to ensure stable hypnosis. If depth of anesthesia was insufficient, defined by a rise of BIS over 60, an additional bolus of propofol was administered (0.25 mg kg-1). If anesthesia was still insufficient, a single bolus of the study drug was administered (10 µg kg-1 alfentanil or 0.1 µg kg-1 sufentanil). Occurrences of patient movement, raise of mean arterial blood pressure (MAP) or of heart rate to more than 30% over baseline were also considered signs of insufficient depth of anesthesia.
Bradycardia below 45 min-1 was treated with atropine 0.25 mg. Hypotension, defined as MAP lower than 30% below baseline, was treated with repeated boluses of 0.1 mL Akrinor ® (mixture of cafedrine and theodrenaline, in Germany drug of first choice for treatment of intraoperative hypotension).
The continuous administration of the study drug was stopped as soon as subcutaneous suturing of the skin began. Propofol was stopped at the end of suturing. Positive-pressure ventilation was continued until the patients opened their eyes on command. The laryngeal mask was removed when extubation criteria were met: spontaneous movement, sufficient spontaneous breathing and opening of the mouth on command.
Patients were observed for two hours in the post anesthesia care unit (PACU). Postoperative pain intensity in the PACU was measured with a visual analogue scale (0-100). If the pain scores exceeded 30, or if patients showed signs of discomfort from insufficient analgesia, the patients were offered additional pain relief (piritramide 3 mg intravenously, equivalent to approximately 2 mg of morphine).
Nausea and vomiting were classified as none, mild, moderate or severe. These observations as well as heart rate, blood pressure and peripheral oxygen saturation (SpO2) were recorded 0, 30, 60, 90 and 120 minutes after arrival in the PACU. Treatment of nausea and vomiting was to be done according to the established standards of our institution.
Propofol used for maintenance was calculated from overall propofol use minus induction bolus. The effect-site levels of the opioids were calculated with STANPUMP (5, 6), using the pharmacokinetic parameters found by Maitre et al. for alfentanil (7) and the parameters published by Gepts et al. for sufentanil (8) and propofol (9). The data from the patient monitor was collected with automated data acquisition software (10).
All values were entered into a database (Microsoft Access, Microsoft, Redmond, WA, USA). Statistical calculations were performed with SPSS for Windows (SPSS Inc., Release 16.0.2) using Pearson’s Chi-Square for binominal outcomes, the Student’s T-test for normal distributed values, and the Wilcoxon-Mann-Whitney test for the comparison of other distribution values. The Kruskal-Wallis-Test was used to compare ordinal outcomes (nausea and vomiting). A value of P < 0.05 was considered significant.
4. Results
38 patients were included in the study. In two cases technical difficulties with the BIS-monitor occurred. Two patients were excluded because surgery was postponed; another two had suspicious intravenous lines during surgery (accidental removal of the line by the surgeon and extravasation, respectively) and were unblinded for safety reasons. The data of the remaining 32 patients were analyzed.
4.1. Baseline Demographics
There were no differences between the study groups regarding age, height, weight or duration of anesthesia (Table 1). Baseline values for MAP and HR showed no significant differences.
| Alfentanil Group | Sufentanil Group | P Value | |
|---|---|---|---|
| Age, y | 54 ± 13.6 | 51 ± 8.1 | 0.297 |
| Weight, kg | 67 ± 7.4 | 71 ± 9.9 | 0.106 |
| Height, cm | 166 ± 6.9 | 165 ± 4.4 | 0.104 |
| MAP, mmHg | 107 (96-110) | 109 (103-119) | 0.220 |
| HR, min-1 | 76 (70-83) | 79 (74-88) | 0.213 |
a Values given as median ((quartiles) or Mean ± SD; P < 0.05 considered statistically significant).
4.2. Intraoperative Values
We did not find a difference in intraoperative MAP and HR between the two groups, and also the BIS values had no statistically significant difference. Bradycardia occured significantly more often in the alfentanil group.
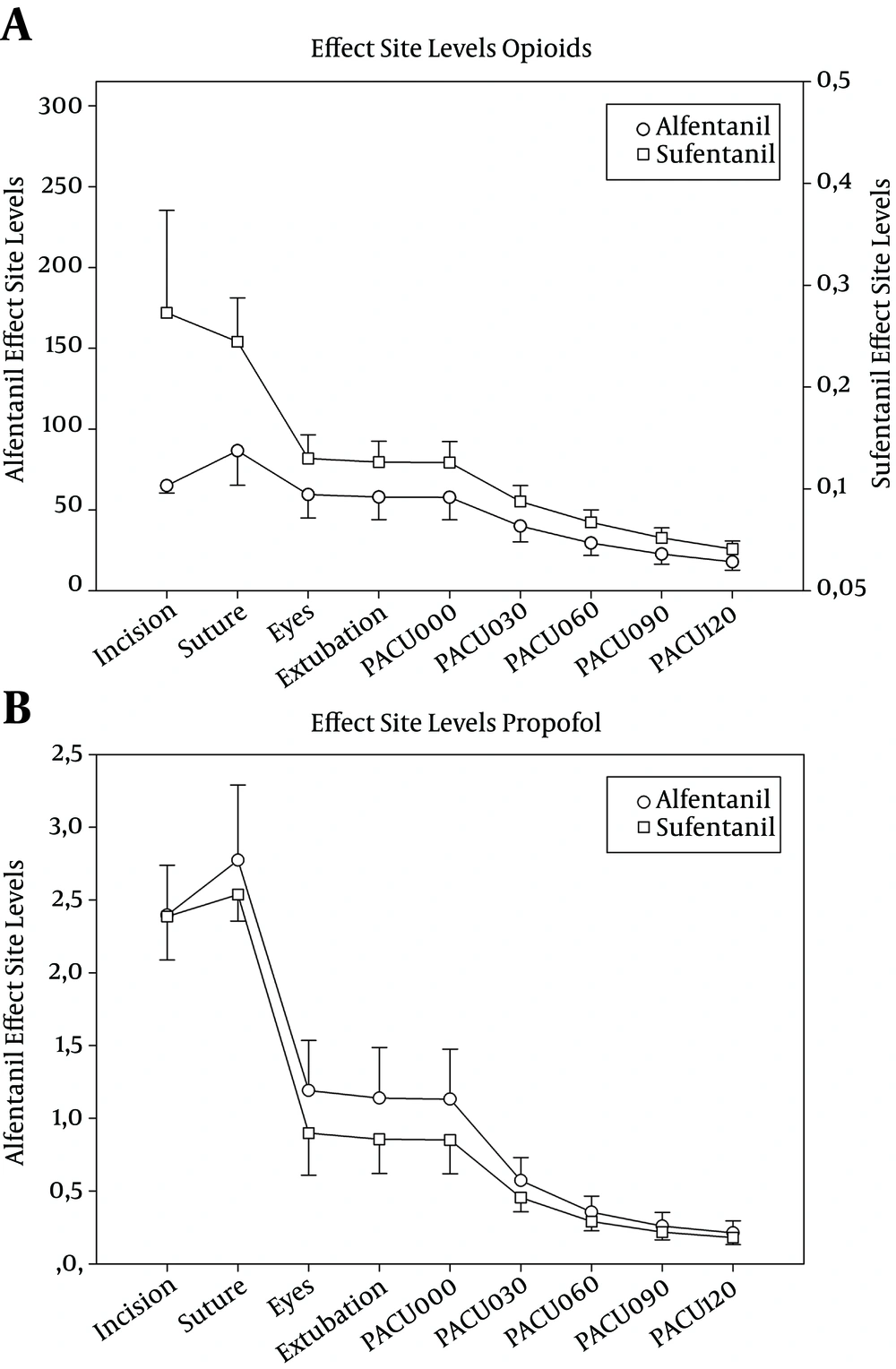
Although the maintenance dose of propofol was not different in combination with either alfentanil or sufentanil (6.6 vs. 6.2 mg kg-1 h-1) the number of additional propofol boluses was significantly higher in the alfentanil group (4.5 vs. 1.6), as well as the number of additional opioid doses needed (0.8 vs. 0.1; Table 2). The mean time to extubation (TTE) in the alfentanil group was 4 minutes shorter than in the sufentanil group, but the difference was not statistically significant. The predicted effect-site levels of sufentanil, alfentanil and propofol are shown in Figure 2.
| Alfentanil Group | Sufentanil Group | P Value | |
|---|---|---|---|
| Duration of drug infusion, min | 84 (48-138) | 77 (54–123) | 0.865 |
| Total Propofo dose, mg | 751 (530–1,108) | 741 (598–950) | 0.940 |
| Propofol for maintenance, mg·kg-1·h-1 | 6.3 (5.9-7.5) | 6.1 (5.7–6.6) | 0.243 |
| Patients requiring additional propofol, n | 14 (88%) | 8 (50%) | 0.022 |
| Patients requiring additional opioid, n | 4 (38%) | 1 (6%) | 0.033 |
| Bradycardia, n | 6 (38%) | 1 (6%) | 0.033 |
| Hypotension, n | 8 (50%) | 8 (50%) | 1.000 |
| Time to eye opening, min | 6 (3–6) | 9 (3–15) | 0.138 |
| Time to extubation, min | 6 (4–10) | 10 (4–16) | 0.239 |
a Values given as median (quartiles) or mean ± standard deviation; P < 0.05 considered significant).
Effect site levels of the opioids (ng mL-1) and of propofol (µg mL-1), calculated by pharmacological modelling (mean ± standard deviation). Timepoints are begin of surgery (“Incision”), begin of suture (“Suture”), eye opening (“Eyes”), extubation and PACU stay, zero to 120 minutes (“PACU000” “PACU120”). Alfentanil and Sufentanil levels are scaled by equipotency (1:630). Opioid plasmalevels are statistically significantly different at every timepoint (P < 0.001; T-Test). Difference of propofol plasma levels is statistically significant from “Eyes” through “PACU030” (P < 0.05).
4.3. Postoperative Care Unit (PACU)
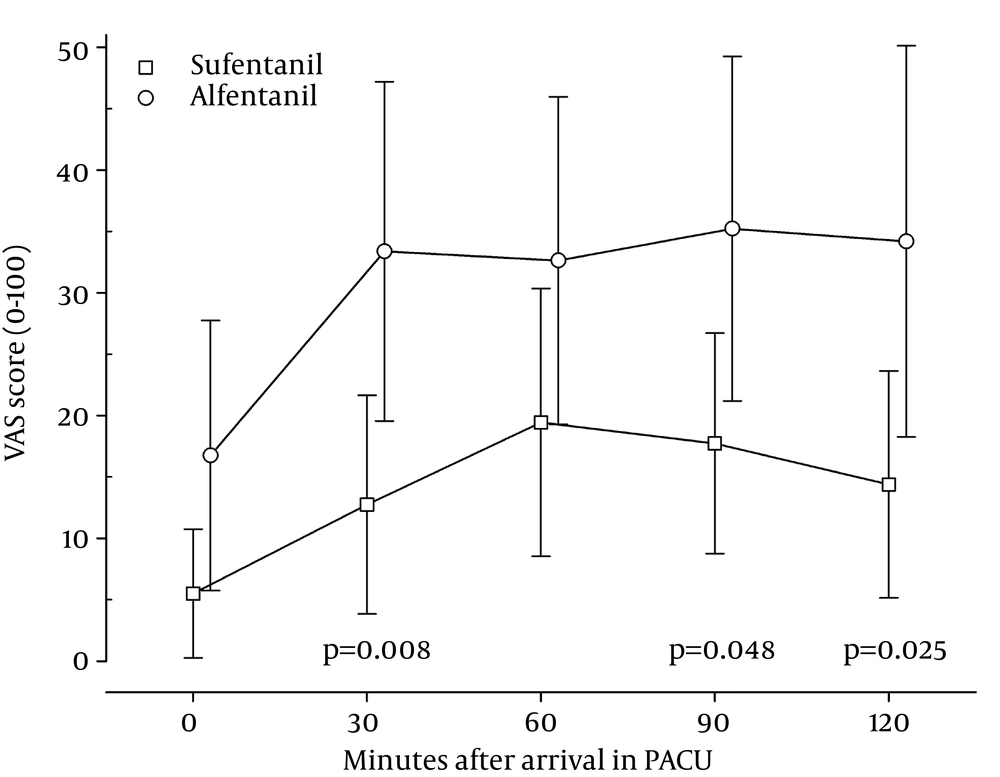
Patients recovering from anesthesia with sufentanil had lower mean and lower maximum pain scores throughout the whole stay in the PACU (Figure 4). Differences were statistically significant at 30, 90 and 120 minutes after arrival. The majority of the patients receiving sufentanil reported a VAS score of 0 during the first two interviews in the PACU, and remained at 20 or lower for the remaining observation time. The need for additional pain medication in the PACU was also significantly different between the two groups (Table 3). 44% of the patients in the alfentanil group needed additional pain treatment versus 13% in the sufentanil group. There were no statistically significant differences in the occurrence of postoperative nausea and vomiting between the groups. All patients were awake and alert on the way to the PACU and remained so during their PACU stay.
| Alfentanil Group | Sufentanil Group | P Value | |
|---|---|---|---|
| Patients needing opioid analgesia, No. (%) | 7 (44) | 2 (13) | 0.049 |
| Total piritramide dose, mg | 0 (0–3) | 0 (0–0) | 0.044 |
| Nausea, no/mild/moderate/severe | 14/2/0/0 | 14/2/0/0 | 1.000 |
| Vomiting, no/mild/moderate/severe | 16/0/0/0 | 14/2/0/0 | 0.151 |
a Values are given as median (quartiles) or mean ± standard deviation; nausea and vomiting as number of occurrences. P < 0.05 considered statistically significant.
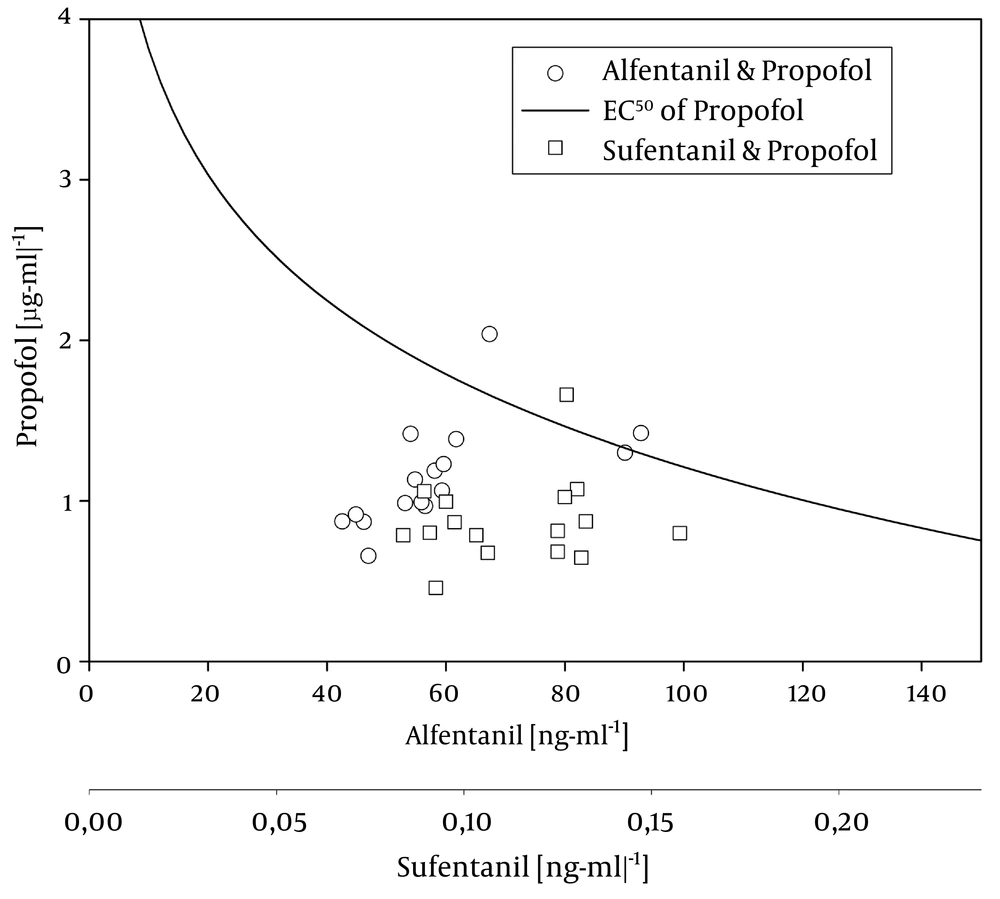
Plasma level pairs of propofol (µg mL-1)/alfentanil (ng mL-1) and propofol (µg mL-1)/sufentanil (ng mL-1 Sufentanil axis is scaled according to estimated equipotency (630:1). The curve represents the plasma level combinations associated with a 50% probability of regaining consciousness according to Vuyk et al.: EC50 of propofol = 6.4194 – 1.1310 x Ln Calf (20).
5. Discussion
In this study we compared a standardized protocol for TIVA with either sufentanil or alfentanil. We found that both opioids provide stable intraoperative hemodynamics and fast emergence from anesthesia. The incidence of PONV was equally low in both groups, but those patients who had received sufentanil reported lower pain scores and needed less additional analgesics in PACU.
Clinicians use these opioids under the assumption that the equianalgesic dose ratio is 100:1 for alfentanil versus sufentanil, an assumption that in fact is supported by some studies (3, 4). Reviewing published data, we found a wide range of equipotency estimates from 40:1 (11, 12) up to 300:1 or even 500:1 (13, 14). This might be due to the fact that the term equipotency is not clearly defined. It has been used for comparison of single bolus injections as well as for plasma and effect-site levels, and methods for its assessment range from MAC reduction of volatile anesthetics to comparisons of EEG depression.
Shafer and Varvel published an in-depth comparison of the pharmacokinetics and dynamics of fentanyl, alfentanil and sufentanil, estimating an equipotency by effect-site levels of 1:630 for alfentanil vs. sufentanil (15). Based on this ratio, the predicted effect-site levels of sufentanil were approximately twice as high as those of alfentanil during the surgical case (incision to suture, Figure 2), suggesting an effective equipotency ratio of the administered drug of 200:1. This is supported by the observation that the patients in the group receiving alfentanil did have a higher demand for additional propofol and opioid boluses.
In their 1992 landmark study, Hughes et al. coined the term “context sensitive half-time” as a consequences of their finding that the half-time of drugs that are redistributed depend on the duration of administration (16). According to the data from their paper, the context sensitive half-time of sufentanil is less than half as high as the half-time of alfentanil (for duration administration of approximnately 1-7 hours). This could explain the much steeper decline in the effect-site level of sufentanil than the elevl of alfentanil between the timepoint “suture” (when the continuous infusion was stopped) and “eyes” (Figure 2).
After Eye Opening the effect-site levels are still 40% higher, and the effect-site levels of both opioids levels decline steadily at the same rate throughout the PACU stay (PACU000 through PACU120). Nevertheless, scaled by equipotency at the effect-site as stated above (1:630), the sufentanil levels are significantly higher throughout the whole study period (P < 0.001).
5.1. Stability of General Anesthesia
Due to central sympathicolysis, all opioids are negatively chronotropic (17, 18) and can cause hypotension by peripheral vasodilatation. Sufentanil is believed to have the least adverse effect on hemodynamics (12, 19). Even though the relative effect-site levels of sufentanil were higher, there was no difference in occurrence of hypotension (Table 2), and the patients in the alfentanil group had a significantly higher incidence of bradycardia than those who received sufentanil.
5.2. Time to Extubation
The mean time to extubation was 4 minutes shorter after alfentanil, which was statistically not significant (Table 2). Additionally, a short delay is probably not clinically significant and can easily be compensated by stopping the opioid infusion earlier. Studies with markedly larger amounts of opioids have not found any difference in awakening times either (15, 20, 21). Most probably the duration of the clinical effect is based not only the difference in context sensitive half-times, but also the different affinity to the µ-receptor.
Vuyk et al. provided a formula to calculate the 50% probability of regaining consciousness (20). The resulting function is plotted into Figure 3 together with the predicted plasma levels at extubation of our patients. We found that nearly all of our patients had an opioid/propofol level combination well below the predicted threshold when they opened their eyes. By definition, we would have expected to find 50% of the data above and below the threshold. This unexpected finding is probably due to the additional analgesic effect of the diclofenac that had been administered preoperatively to all our patients, and the additional effect of the oral midazolam premedication which the patients had received on the morning of the procedure.
5.3. Postoperative Analgesia
The patients in the sufentanil group had lower VAS scores throughout their time in the PACU (Figure 4). In our study, we cannot distinguish between a higher intrinsic analgesic potency of sufentanil as compared to alfentanil due to the different effect-site levels. But the low VAS scores throughout the PACU stay and the lesser need for additional analgesics suggest that sufentanil has a strong residual analgesic effect.
5.4. Postoperative Nausea and Vomiting (PONV)
Most patients did not report any nausea during their stay in the PACU (Table 3). Only two patients in each group reported mild nausea, and two patients in the sufentanil group experienced a single event of vomiting. No patient required treatment.
There was not statistically significant difference in the occurrence of PONV. This corresponds with the results of Bloomfield et al. who studied different dosing schemes and found no difference in PONV incidence following alfentanil and sufentanil analgesia (21).
5.5. Limitations
An important implication of clinical protocols for surgical procedures is the recovery time, defined both by recovery scores and as the time when the patient is ready to be discharged from the PACU (22). When our study was performed, PACU nurses in our institution did not routinely assess and document recovery scores, and there were no standardized discharge criteria in place. Additionally, discharge from PACU depends on multiple other factors (e.g. availability of patient transport home or an inpatient bed), making it difficult to evaluate the direct impact of the anesthesia technique. However, all of the patients in our study were fully awake, alert and oriented when they were transferred to PACU. Hence we would not expect a significant difference in discharge criteria between the two study groups.
Another limitation of our study is the relatively small sample size. With a larger number of patients, we would expect the confidence intervals to become smaller, potentially yielding an even more pronounced difference between the pains scores in the PACU. To fully understand the relevance of such a protocol in daily clinical practice, we would need to perform a cohort study over a sufficiently long amount of time with a large number of patients.
Our protocol for TIVA with propofol and either sufentanil or alfentanil provides stable and safe analgesia for general anesthesia during moderately painful surgery. However, the protocol with sufentanil provided better postoperative pain control. As sufentanil is also less expensive than alfentanil, we would prefer sufentanil as the opioid of choice for a standardized TIVA regimen. Additionally, based on the amount of propofol needed for maintenance (Table 2), we suggest that propofol should be administered continuously with a minimum rate of 6 mg kg-1h-1.