1. Background
Nowadays using central venous catheters is of great importance in medical care of critically ill patients (1). Central venous catheter is a device for accessing the central veins and used for hemodynamic monitoring, parenteral nutrition, chemotherapy, hemodialysis etc. (2). The use of central venous catheters had a great role in medicine and has led to a reduction in duration of hospitalization, increment of safety and reduction of hospital charges (3, 4). Annually more than five million central venous catheters are used in the United States of America to measure the hemodynamic variables that cannot be assessed using noninvasive methods (5, 6). More than 15% of patients in whom a central venous catheter is inserted face the complications of catheter including mechanical complications (5-19%), infectious complications (5-26%) and thrombotic complications (2-26%) (5, 7). Besides, catheter associated complications are one of the reasons and an important reason for patients mortality (3, 8). Prevention of catheter occlusion is necessary for its appropriate functioning and longer use for giving care to patients. Nowadays, nurses irrigate central venous catheters routinely to maintain their patency (9, 10). The solution used for irrigation of catheter includes 0.9% sodium chloride and heparinized saline (11, 12). Concentration of heparin in this solution differs from 10 to 1000 units in milliliters (13, 14). Nonetheless, using heparinized saline as the standard solution causes high charges for patients and medical care centers and increases the risk of thrombocytopenia in patients. Heparin induced disorder occurs in almost 30% of patients (15-17). According to conducted studies, there is a possibility for occurrence of thrombocytopenia or thrombosis even 40 days after cessation of heparin (18-20). Other risks of using heparin include allergic reactions and bleeding (19, 21, 22). Since patient’s safety is the highest priority of medical care, the need for replacing heparin with a safer drug with the same efficacy seems necessary.
2. Objectives
Since normal saline solution with least complications can be one of the options in preventing catheter associated complications and because the conducted studies reported controversial results, this study was performed to compare the effect of heparin with 0.9% sodium chloride solution in keeping the central venous catheters open.
3. Patients and Methods
This was a double-blind randomized clinical trial. The sample of research consisted of all patients hospitalized in ICU of Roohani Hospital of Babol city who needed central venous catheter during March 2013 to February 2014. The sample size was calculated using the results of Rabe’s study (22) with a confidence interval of 99% and considering a possibility of 42 patients exiting the study in each group. The inclusion criteria for the study included 18-60 years of age, time passed from the insertion of catheter less than 12 hours, usage of triple lumen silicone catheters, patient’s blood platelet of 150000-450000, PT (Prothrombin Time) of 11-12.5 seconds, PTT (Partial Thromboplastin Time) in the range of 35-45 seconds and received one liter of serum KVO (keep vein open) during 24 hours (13, 23, 24). The exclusion criteria included risk of bleeding, receiving blood products and TPN (Total Parenteral Nutrition) during study, and an increase in body temperature higher than 37.7 ºC (13, 23, 24). Sampling was performed by random sampling method. The patients who fulfilled the inclusion criteria were divided into two groups using random numbers generated by Excel software’s Rand Between Function. Subjects were included after signing a consent by themselves or their family (in patients with low levels of consciousness or comatose patients). After choosing patients, the researcher recorded their demographic data and explained the necessary details about the methods of study. Insertion of catheters was performed in ICU by an anesthesia specialist in aseptic condition in subclavian or jugular areas (17, 23). In the heparin group, heparin (Alborz Drug Company, Iran) with the product number of 1228129919 was used. The solution was prepared by a 5000 unit heparin ampoule added to a half liter of normal saline, hence each milliliter of the prepared solution contained 10 units of heparin. After injection of drugs to patients, 3 mL of the solution was injected as well. In normal saline group patients, 10 mL 0.9% sodium chloride was injected in each lumen of the catheter (13). Patients were unaware of the used method. Catheters were examined by the researcher for blood return and flushing every 48 hours using patients medical data sheets (13). In this study, the ward nurse prepared heparin and normal saline solutions and the researcher was unaware of the content of serum. During the examination of catheters, all patients were lying on their backs. If flushing or taking blood sample from the catheter was not possible, it was considered non-functional and removed. The maximum time of study was 21 days and the collected data during these 21 days were recorded every eight hours in the previously prepared checklist. At the end of study, patients’ data were analyzed by SPSS software version 20. In this study for describing the features of research units, descriptive statistics (mean, standard deviation and distribution frequency) were used. For analyzing the data, Kaplan Meier survival analysis, log rank test and Cox regression were performed and for comparing the ratios, the chi square test was used. P < 0.05 was considered significant.
4. Results
In this study, despite random allocation of patients into two groups, no significant difference regarding age, gender, underlying diseases and present risk factors between the groups was observed. None of the patients in the two groups had signs of allergy or local reactions. The mean age of heparin group was 50.0 ± 8.9 and in the normal saline group was 51.98 ± 7.8. Independent t-test showed no significant difference between the two groups regarding age (P = 0.302).
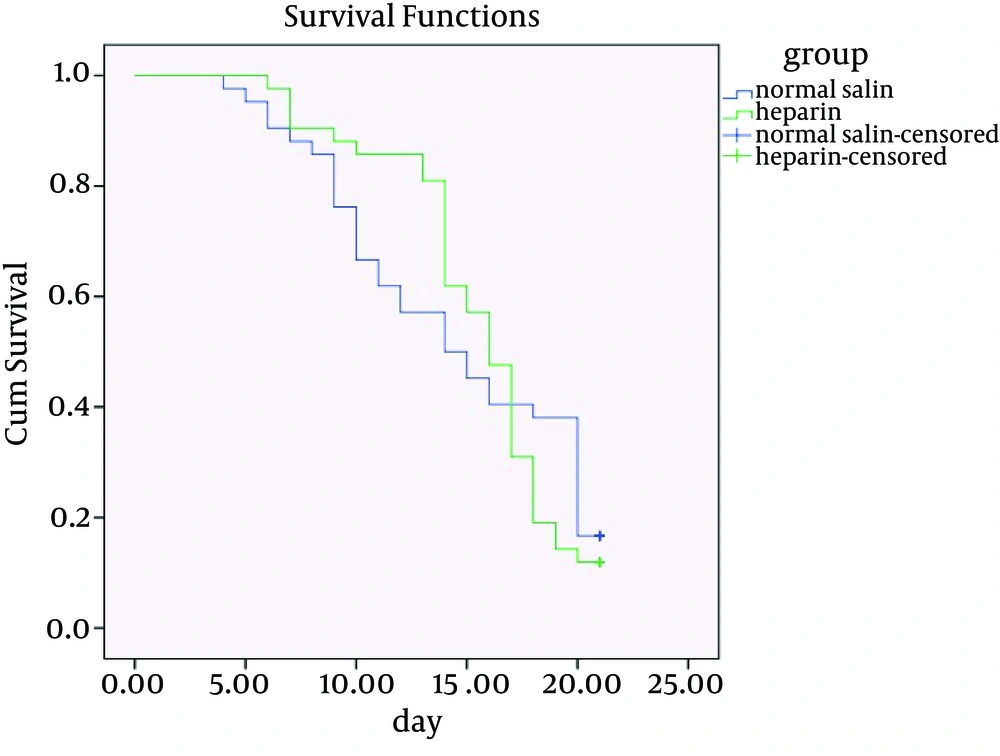
In normal saline receivers, 52.4% were male and 47.6 females. Besides, 52.4% of heparin saline receivers were males and 47.6% females. There was no significant difference in gender of the two group using chi-square test (P = 1). Mean and standard deviation of number of days of flushing were 14.45 ± 5.56 in the normal saline group and 15.47 ± 3.9 in the heparin group. Cox regression test showed no statistically significant difference in flushing between the two groups (P = 0.872). In addition, the possibility of taking blood from the catheters in the normal saline group was 13.8 ± 5.94 days and in the heparin group was 15.23 ± 4.09 days. Cox regression test showed no statistically significant difference in the possibility of taking blood form catheters in the two groups (P = 0.745). Furthermore, Cox regression test showed no statistically significant difference regarding the effects of heparin and normal saline in maintenance of patency of venous catheters in 7 days (P = 0.941), 14 days (P = 0.363) and 21 days (P = 0.872) (Table 1), (Figure 1).
| Number | 7 Days | 14 Days | 21 Days | |
|---|---|---|---|---|
| Heparin | 42 | 6.97 ± 0.15 | 13.04 ± 2.29 | 15.47 ± 3.99 |
| Normal Saline | 42 | 6.83 ± 0.58 | 11.76 ± 3.01 | 14.45 ± 5.56 |
| Total | 84 | 6.90 ± 0.42 | 12.40 ± 2.74 | 14.96 ± 4.84 |
| P Value | 0.941 | 0.363 | 0.872 |
a Data are presented as mean ± SD.
5. Discussion
There was no significant difference between using heparin and normal saline for keeping the catheters open for 21 days. According to the results of this study, normal saline can replace heparin solution for washing catheters, because it does not have side effects and a better choice economically. Comparison of efficacy of flushing with normal saline versus low dose heparin saline (10 units per milliliter) showed no significant difference on maintenance of the patency of the catheter, prevention of its occlusion and catheter survival. This result is consistent with Schallom’s study (23). Parallel with the present study Selleng et al. compared the efficacy of normal saline in keeping the central venous catheter open versus 100 and 500 units of heparin doses in 24 hours, 72 hours and the end of treatment (5 days). The results of this study showed that heparin had no advantage over normal saline (16). Although the duration of this study was shorter than the present study, the results were the same, which shows that the time of intervention does not affect the rate of patency of catheters. In contrast to the results of the present study, Rabe et al. concluded that use of heparin with higher doses (5000 units per milliliter) caused a significant increase in catheter survival rate in 99 patients from Germany (22). In this study, high doses of heparin were used, which was not in line with the results of the present study. Because heparin leads to different side effects and increases the medical charges (22), using it in high doses is not rational medically and economically. Findings of the present research about the primary outcome, which was the possibility of taking blood samples from the central venous catheters, showed no significant difference between the two groups of heparinized saline and normal saline. Parallel to the present study, in Ling’s investigation conducted in neurologic intensive care unit of a general hospital in Singapore for five days, no statistically significant difference in the possibility of taking blood samples was seen (13). Despite the shorter duration of this study than the present study, the results were the same, which shows that the time of intervention does not affect the possibility of taking blood from catheters. Findings of the present study showed that concerning the secondary outcome, which was the possibility of flushing the central venous catheters in the two studied groups, no significant difference between the two groups was observed. These results were consistent with the results of the study performed by Schallom et al. on 341 patients hospitalized in intensive care unit of Barnes Jewish hospital in America for five days. In this study, no significant difference was reported between usage of heparin and normal saline during eight hours (23). In the present study, the time of examining the catheters for flushing was every eight hours, the same as the Schallom’s study (23). Although the present study was performed for 21 days, the results were the same in the both studies. Rabe et al. investigated the effect of high dosage heparin versus normal saline during 48 hours, and found a significant difference compared to the present study (22). This study was performed using higher doses, which can explain the difference in the results of the two studies.
According to the results of this study and other studies, it is recommended to use normal saline instead of routine use of heparin saline solution for washing central venous catheters. Because using normal saline is more beneficial economically and does not lead to side effects caused by heparin, the most important of which is thrombocytopenia.